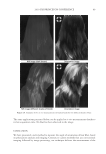
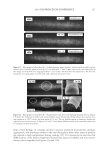
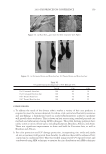
J. Cosmet. Sci., 62, 209–228 (March/April 2011) 209 Anionic/cationic complexes in hair care TONY O’LENICK, Siltech LLC, 2178 Luke Edwards Road, Dacula, GA 30019. Synopsis The formulation of cosmetic products is always more complicated than studying the individual components in aqueous solution. This is because there are numerous interactions between the components, which make the formulation truly more than the sum of the parts. This article will look at interactions between anionic and cationic surfactants and offer insights into how to use these interactions advantageously in making for- mulations. BACKGROUND Products sold into the hair care market are a combination of many individual ingredients that interact with each other often in unpredictable ways. The ability to understand these interactions and to use them to make products with synergistic interactions is one major area of research. One such area of interest is the interaction between cationic and anionic surfactants. Fatty quaternaries have been known for many years. Because of their fatty nature and positive charge, these compounds fi nd application in a variety of areas including as conditioners for hair and skin. Despite the fact these materials have been recognized as key cosmetic additives, there is little published on the structure function relationship on basic properties. For example, some quats are very insoluble when added to anionic surfactant, others have improved compatibility. The ability to select quats that have optimum compatibility with anionic systems offers the formulator fl exibility in formu- lating heretofore unavailable. There is also much confusion related to deposition of cationic material onto hair as measured by a number of red dye uptake tests. These tests merely measure cationic on the surface of the hair. Since deposition on hair made from a solution containing cationic and anionic, contains no free cationic, no red color is observed with these tests. This does not mean however there was no deposition, it sim- ply means the deposited material does not have an overall positive charge and conse- quently does not bind dye. Anyone that has added stearylalkonium chloride to sodium lauryl sulfate and ob- served the white sticky solid that results knows anionic and cationic surfactants can be incompatible. We have begun to call anionic and cationic materials that produce a white gunky solid when mixed together hard complexes. As the expression implies the cationic and anionic compound possess properties which when added together form insoluble complexes (salts). We set out to determine if there are cationic materials
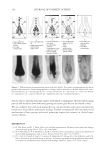
JOURNAL OF COSMETIC SCIENCE 210 having different structures which could be more soluble in the presence of anionic surfactants. The terms used here for quats and anionic materials are an adaptation of the work of Pearson used to describe acids and bases. Pearson proposed that “hard acids bind strongly to hard bases and soft acids bind softly to soft bases” (1). The interaction between anionic and cationic materials has been studied prior to this work (2–4). The structural changes that can be made to cationic molecules can “soften” them, making them more compatible with anionic systems. Alternatively, there should also be the possibility of developing an anionic material that has increased compatibility with cationic surfactants, perhaps a more highly ethoxylated sulfate or a sulfosucci- nate. However, this concept of modifying the anionic, is a topic for another investi- gation. A study was undertaken to determine (1) the compatibility of specifi c quats with SLS and SLES, (2) the foam properties of the combinations with SLS and SLES, (3) the substantiv- ity of these combinations in aqueous and anionic surfactant delivery systems, and (4) the combing force needed. The quats studied conform to the following structure: The preferred defi nitions for the study groups are (Table I): R1 1. Alkyl (C12) 2. Ricinoleylamidopropyl 3. Dilinoleylamidopropyl 4. Cocamidopropyl R2 1. Methyl -CH3 2. 2-hydroxy ethyl -CH2CH2OH R3 1. Methyl -CH3 2. Benzyl -CH2-C6H5 3. Glyceryl -CH2-CH(OH)-CH2-OH (A) COMPATIBILITY WITH ANIONIC SURFACTANTS A determination of compatibility of a variety of quats with two anionic surfactants, so- dium lauryl sulfate and sodium laureth-3-sulfate was made. The compatibility was deter- mined by titration. The point at which an anionic solution containing 10% anionic either became hazy or formed a precipitate was determined.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)