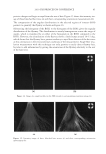
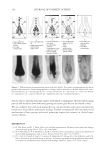
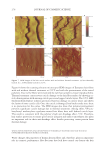
2010 TRI/PRINCETON CONFERENCE 173 It is important to note that the micelles are made up of mixtures of all of the surfactants that are in the shampoo. In fact, it is commonly assumed that the relative amount of each surfactant in a micelle is the same as in the bulk formulation. This means that the average surfactant charge is a convenient measure of the potential that would exist on each mi- celle. Thus, it will be considered to be a theoretical measure of micelle charge density. The relative sizes of the micelles can be estimated from the viscocity of the shampoos it- self. Since the micelles primarily increase in one dimension, a convenient description of micelle size is aspect ratio. This terminology will be used here. An estimate of this value has been obtained in previous work (14) by modeling the change of the Brookfi eld viscos- ity measured at 20rpm with the various surfactant blend composition (in terms of SLES-3, CAPB and SLS content) and Equation 4 summarizes the modeling fi t. Aspect ratio = wt% CAPB + 0.8* wt% SLS + 0.15* wt% SLES 3 + 0.15*(wt% SLS 2.1) *(wt% CAPB 4.1) - - - (4) This measure of aspect ratio is not a pure one. It is partially confounded with the amount of surfactant that is in the formulation as well as the amount of salt. Thus, it should be only considered as a rough estimate of size. These factors will be used to rationalize per- formance. For the silicon deposition data presented above, the micelle aspect ratio and total micelle charge were calculated for each surfactant blend. The ratio of aspect ratio to micelle charge against the total silicone deposition for each cationic cassia polymer is plotted in Figures 13 and 14. It can be seen from the P value in Figure 13 that in the case of the higher cationic charge cassia polymer, there is a relatively high confi dence level (98%) that these factors are important in explaining silicone deposition. The results show that as the ratio of the aspect ratio to micelle charge increases, the silicone deposition with EX-906 decreases, i.e. the conditioning performance decreases. The ratio shown is an indirect measure of the inverse of micelle charge density (as the micelle charge density increases, the silicone deposition increases). Thus, the data show that micelles having a Figure 13. Correlation of silicone deposition with the ratio of aspect ratio to micelle charge for EX-906 (3.0 mEq/g).
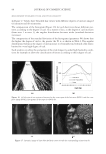
JOURNAL OF COSMETIC SCIENCE 174 high charge density (either through a small aspect ratio or high charge or both) provide a favorable environment for interaction with the higher cationic charge cassia polymer and lead to higher silicone deposition and superior conditioning performance. The effects of the nature of the interaction of high charge density micelles with the higher charge density cationic cassia polymer EX-906 are not well understood. Several explanations are possible. The complex may be more likely to adhere better to the neg- atively charged hair surface due to better neutralization of the high cationic charge polymer with the high charge micelles or the fl occulation may be more effi cient be- tween EX-906 and highly negatively charged micelles to entrap the silicone. It is also possible that there is a different interaction (or conformation) between EX-906 and highly charged micelles compared to micelles with high aspect ratio (rod-like) or lower charge. The ratio of aspect ratio to micelle charge for the lower charge density cationic cassia polymer EX-1086 is plotted against the total silicone deposition and shown in Figure 14. In this case, it is seen that there is not a signifi cant correlation (P values of 0.39) between the silicone deposited on the hair and the ratio of aspect ratio to micelle charge. The mechanism for optimizing the conditioning performance with the lower cationic charge density cassia polymer appears to be different from the higher cationic charge density cassia polymer. Extension of the coacervation curve (the latter part of coacervation curve after dilution 10) may help to enhance performance of EX-1086 but not EX-906. Thus, forming as much coacervate over as wide of a dilution range as possible may lead to better performance. It is hypothesized that an inherent lack of adhesion or poorer fl occulation of the coacervate formed by EX-1086 will lead to lesser deposition. This could then be compensated by a higher amount of coacervate. The silicone deposition data for both EX-906 and EX-1086 can be merged and modeled together if the charge on the cationic polymer is taken into account. This is done as shown in Figure 15. The overall effects of the parameters show that a greater aspect ratio de- creases silicone deposition and a greater charge on the cationic cassia polymer increases it. Figure 14. Correlation of silicone deposition with the ratio of aspect ratio to micelle charge for EX-1086 (1.7 mEq/g).
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)