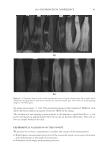
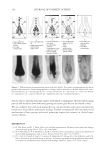
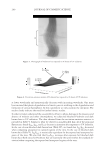
2010 TRI/PRINCETON CONFERENCE 163 EXPERIMENTAL MATERIALS Cassia gum is a natural vegetal carbohydrate based on mannose and galactose sugars extracted from the endosperm of the seed of cassia tora and cassia obtusifolia. It is a member of the ga- lactomannan family of polysaccharides with a ratio of mannose to galactose content of at least 5:1. Cassia grows wild in tropical zones around the world. Cassia has been used for over a thousand years in Ayurvedic and Chinese medicine to treat skin ailments, indigestion, and pain. It is also used today as a gelling agent in pet and human food applications. The chemi- cal structure of cassia gum is illustrated in Figure 2. Polysaccharide derivatives have a long history of use in personal care applications as thickeners, conditioning polymers, deposition aids and fi lm formers. Cationic derivatives of guar gum, another galactomannan, have been successfully used in conditioning shampoos in combination with silicones to impart im- proved combing and sensory properties. Cassia gum can be modifi ed to generate cationic galactomannans with various levels of cationic substitution (9). That modifi cation produced two novel cationic cassia conditioning polymers, EX-906 and EX-1086, with cationic charge density levels of 3.0 mEq/g, and 1.7 mEq/g, respectively, available from Lubrizol Advanced Materials, Inc.). The INCI classifi cation for these polymers is cassia hydroxypropyltrimonium chloride. The chemical structures for EX-906 and EX-1086 are represented in Figure 3. The surfactants used in this study are sodium lauryl ether sulfate (SLES-3), sodium lauryl sulfate (SLS) and cocamidopropyl betaine (CAPB), all commercially available from Lubrizol Advanced Materials, Inc. METHODS Clarity measurements. The clarity of a formulation is measured in %T (% transmittance) at 420 nm by a Brinkmann PC 920 colorimeter. Clarity measurements are referenced against de-ionized water (clarity rating of 100%). Hair tress washing procedure. Virgin European brown hair tresses (2.5 g per tress) are pre- washed with a surfactant (10 wt% sodium lauryl sulfate) and thoroughly rinsed. Two-in- one conditioning shampoos (0.25 g) prepared with the formulations of the design of experiments are applied to each hair tress and gently lathered for 1 minute with 40 Figure 2. Chemical structure of cassia gum.
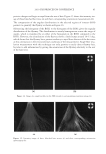
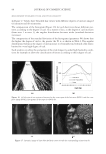
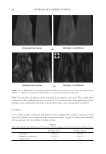
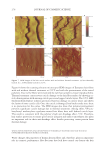
JOURNAL OF COSMETIC SCIENCE 164 strokes and subsequently rinsed under fl owing tap water (6.0 l/min) at 37° ± 2°C for 30 seconds. The tresses are shampooed a second time and rinsed as previously described. After rinsing, the tresses are dried at 23° ± 1°C and 50° ± 5% relative humidity. Cationic polymer deposition on wool. Cationic polymer deposition is measured by using the Direct Red 80 dye colorimetric test (10). The cationic polymer deposition on a virgin wool swatch was studied after two washes, using the washing procedure previously de- scribed. Three wool swatches per experimental shampoo are washed two times with 0.25 g of shampoo, immersed into a dilute solution of Direct Red 80 dye for one minute and rinsed copiously to remove all excess dye. The intensity of red coloring (a*) is measured with a spectrophotometer (Labscan XE, HunterLab). Three readings per wool swatch are recorded. Silicone deposition measurement. The relative amount of silicone (silicon atoms) deposited on Virgin European brown hair tress samples from a two-in-one shampoo composition is measured by X-Ray fl uorescence (XRF) spectroscopy (11). A wavelength dispersive XRF spectrometer (PANalytical Axios Advanced Sequential 4kW spectrometer – Model Number PW4400) interfaced with a SuperQ 4 software application and fi tted with a rhodium tube with an InSb crystal utilized to facilitate high sensitivity detection of sili- con corresponding to the Si K alpha band. The samples are analyzed using a qualitative program to measure intensities across a two-theta scan range from 139.75° to 147.99° with a peak maximum at 144.53°. The samples are scanned in a vacuum environment using a tube voltage of 25 kV and a current of 160mA. The scanning speed is 0.05°.s−1. X-rays from the instrument excite silicon atoms deposited on the surface of the hair tress causing them to emit energy and fl uoresce. The silicon fl uorescence is detected and Figure 3. Chemical structure of cationic cassia polymers EX-906 (3.0 mEq/g) (A) and EX-1086 (1.7 mEq/g) (B).
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)