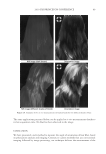
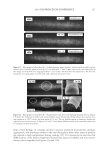
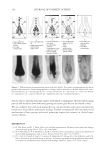
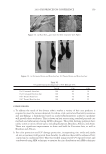
2010 TRI/PRINCETON CONFERENCE 219 Table X Substantivity of Titrated Quat Solutions in SLS Result Tress sample Treatment Positive Negative A Test — No color change B Test — No color change C Test — No color change D Test — No color change E Test — No color change F Test — No color change G Test — No color change H Test — No color change I Test — No color change J Test — No color change K Test — No color change L Test — No color change M Test — No color change N Test — No color change O Positive control — No color change P Negative control — No color change Table XI Substantivity of Titrated Quat Solutions in SLES Result Tress sample Treatment Positive Negative A Test — No color change B Test — No color change C Test — No color change D Test — No color change E Test — No color change F Test — No color change G Test — No color change H Test — No color change I Test — No color change J Test — No color change K Test — No color change L Test — No color change M Test — No color change N Test — No color change O Positive control — No color change P Negative control — No color change
JOURNAL OF COSMETIC SCIENCE 220 No substantivity was observed (Tables X, XI) when quat solutions were delivered from a 10% active, anionic solution of surfactant (SLS and SLES). The test measures cationic deposition as opposed to deposition of a compound of any nature. Since the quat and anionic form a com- plex, the deposited material is not cationic and consequently does not provide a color when tested with the dye test. More representative of the deposition is combing force. (E) INSTRUMENTAL ANALYSIS OF COMBING FORCE PURPOSE Determine the force needed to comb wet and dry hair tresses treated with 0.5% active quaternium compound by instrumental analysis. PROCEDURE Treat hair tresses (1) Treat hair tresses by soaking them in 10-15ml of 0.5% active quat solution for two minutes at 20°-25°C. (2) Rinse hair tresses under running tap water (2.0-2.5 gallons/min, 35°–40°C), for one min- ute. Allow hair tresses to air dry for 24 hours at 20°–25°C and 40-50% relative humidity RESULTS The instrumental analysis of 0.5% active quat compounds (Tables XII, XIII, p. 221) showed that blue (MMM), performed the best followed by yellow (MMG), black (deion- ized water), and red (polyquaternium 10). CONCLUSIONS Quaternium compounds can be classifi ed as hard or soft by their ability to form gelled systems with anionic systems. Cationic systems that form a gel at near stoichiometric amounts are classifi ed as “soft”, those that form precipitates of haze without appreciable viscosity build are classifi ed as “hard” quats. “Soft quats” can produce foam in the systems they gel, albeit at levels below the volume of foam generated by the anionic per se. Quaternium compounds titrated with sodium laureth sulfate (SLES) produced greater viscosities with amido quats. The exception was amido quats containing a benzyl group, which exhibited a low viscosity in SLES. Compounds that contained a benzyl group, or were a alkyl rather than amido, (i.e. AMB, AME, AMG, AMM, AEB, AEG), precipitated at lower levels of titration and are conse- quently classifi ed as “hard quats.” Overall, all quat/anionic solutions tested had less foam than when the anionic itself was tested. This was true for both SLS and SLES. With the exception of quats AEG, AMG, and CaMG, and the negative control, all 0.5% active, aqueous solutions of quaternium compounds produced positive results for cationic substantivity, when evaluated per se. In aqueous solutions of anionic surfactants, all quat solutions, including the positive con- trol (polyquaternium 10), produced negative results. This is thought to be due to the fact
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)