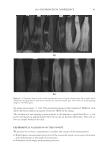
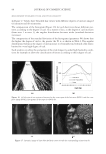
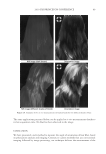
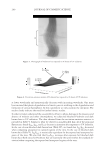
2010 TRI/PRINCETON CONFERENCE 273 collect IR spectra pixel by pixel. By sectioning hair, and collecting spatially resolved in- frared spectra of hair samples, spatially resolved images of the changes in hair protein structure as a result of thermal stresses to the hair were generated. Figures 4a and 4b show the typical IR spectra and the second derivative analysis of a random location in the cortex of undamaged European dark brown hair from 1480-1700 cm−1(Amides I and II) and 3000- 3700 cm−1(Amide A) spectral regions. Bands from 1480- 1700 cm−1 region are sensitive to changes in the protein secondary structural conforma- tion. In order to get the resolutions of the IR bands, secondary derivative analysis was used to locate the different protein peak positions under the curve. The second derivative curve displays the minor component of β-sheet and a major α-helical structure under the curve for undamaged hair. Amide II at 1548 cm−1 is assigned to α-helical structure and Amide II at 1516 cm−1 is assigned to β-sheet conformation (11.). The radio of β-sheet peak intensity to the α-helix band intensity was used to quantify the additional conver- sion of α-helix to β-sheet conformation from thermal treatment. An increase in the ratio indicates an increase in β-sheet composition or a decrease in α-helix content correspond- ingly, and if the ratio remains the same as the undamaged hair, there will be no change in the two components. The ratio maps of β-sheet peak intensity to the α-helix band intensity of hair cross sec- tions are shown in Figure 5a. The ratio bar at the right side with higher numbers and corresponding colors indicates the relative β-sheet intensity. It can be seen that the outer layer of hair has a higher β-sheet level than inside the hair as indicated by the brighter color in the outer layer of the hair cross section. Moreover, the β-sheet content becomes more pronounced in the outside layer of thermally treated hair due to the heat of the iron affecting this part of the hair fi rst. Pretreatment with all three tested polymers tested Figure 4. IR spectra and their second derivative curves of undamaged European dark brown hair. a. Amide I & II region (1480–1700 cm-1), b. Amide A region (3000–3700 cm-1).
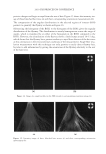
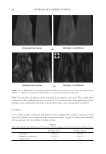
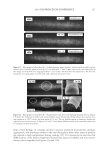
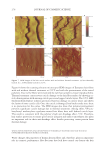
JOURNAL OF COSMETIC SCIENCE 274 effectively prevented the conversion of α-helix structure to the β-sheet conformation. HEC pretreatment provided slight protection to β-sheet conversion. Protein helices are held together by hydrogen bonds between the carbonyl oxygen of amide bonds in the main chains with the imido hydrogen of amides. The Amide A band (N-H stretching) at ~3290 cm−1 is very sensitive to the disruption of hydrogen bonding. When some of the helix unfolds and changes to the extended protein chain or β-sheet conformation, the hydrogen bonds will break, leading to the shift of the Amide A band. Figure 4b shows the IR spectrum of Amide A region and its second derivative curve. The second derivative curve of the Amide A region shows bands at 3292 cm−1 and 3200 cm−1 which are assigned to the trans-bonded and cis-bonded N-H stretching bands, respec- tively. The cis-bonded Amide A band is attributed to the interruption of hydrogen bond- ing due to helix unfolding. To compare the changes in the trans-bonded structure to the cis-bonded structure after thermal treatment, the ratio of the peak intensity at 3200 cm−1, which is attributed to the cis-bonded structure, to the peak intensity at 3292 cm−1, which is attributed to the trans-bonded structure, is used to quantify the additional conversion of trans-bonded Amide A to cis-bonded Amide A structure due to thermal treatment. An increase of the ratio will indicate the increase of cis-bonded component and a decrease of trans-bonded structure correspondingly and if the ratio remains the same as the undam- aged hair, there will be no change in the two components. The ratio maps of cis-bonded Amide A structure to trans-bonded Amide A structure over hair cross sections are shown in Figure 5b. The ratio bar at the right side with higher numbers and corresponding colors indicates the relative cis-bonded Amide A content. Consistently, the content of cis- bonded amide A for thermally treated hair increases after heat exposure. The increase of cis-bonded A content is consistent with the increase of β-sheet formation as stated above. This results confi rms the disruption of the hydrogen bonding structure of helical protein and suggests the unfolding of some helical structure. Pretreatment with all three poly- mers tested effectively prevents the formation of cis-bonded amide A protein bands. Therefore the IR image analysis results are consistent with the DSC results on the ther- mal protection effect of polymers. Figure 5. IR images of thermally treated hair cross section at 232°C with and without polymer pretreat- ment. (a) The ratio maps of b-sheet peak intensity to the a-helix band intensity. (b) The ratio maps of cis- bonded Amide A band intensity at 3200 cm-1 to that trans-bonded Amide A at 3292 cm-1. Dark brown European hair.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)