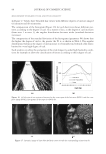
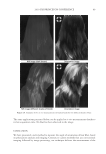
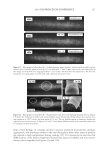
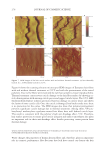
2010 TRI/PRINCETON CONFERENCE 231 the biochemical basis for the cross-linking process was assisted by the discovery that it is manifested in cultures of human epidermal cells (14). Requiring elevated calcium ions for their enzymatic activity, transglutaminases participate in the terminal stage of keratino- cyte differentiation. A major form encoded by the gene TGM1, defi cient in a large per- centage of lamellar ichthyosis cases, is located on the inside of the plasma membrane and is thereby positioned to form the cross-linked envelope at the cell periphery (15). Defects in the protein envelope are envisioned to perturb the attachment to it of the lipid barrier. Consequences of an improperly formed lipid barrier include entry of exogenous hydro- philic agents and excessive transepidermal water loss through the epidermis. Mature corneocytes consist largely of hydrophobic keratin intermediate fi laments and keratin associated proteins held together by disulfi de bonds. These components are further stabilized by proteins cross-linked to them by transglutaminase activity. In addition to the TGM1 gene product at the cell periphery, which incorporates membrane-bound and junctional proteins, a transglutaminase in the cytoplasm encoded by the TGM3 gene is available to incorporate soluble proteins (16). Our recent proteomic efforts analyzing the corneocyte have focused on these disulfi de- and isopeptide-linked components. Thus, samples of hair, nail or callus are fi rst rinsed in detergent (2%SDS) to remove soluble protein, generally negligible in amount, and adherent adventitious material to yield the sample to be analyzed. METHODOLOGICAL APPROACH Our strategy has been to separate the material solubilized in SDS dithioerythritol (DTE) from the remainder resistant to solubilization due to isopeptide bonding. To facilitate re- lease and removal of the solubilized protein, the hair was extracted fi ve times, as shown in Figure 2. Each time, it was incubated overnight at 70°C in 2% SDS - 0.1 M sodium Figure 2. Solubilized protein from human hair shaft, nail plate, or epidermis extracted in 2% SDS - 20 mM DTE - 0.1 M sodium phosphate (pH 7.8). Each extract of hair and nail consisted of daily incubation for ≈ 22 hr at 70°C and then magnetic stirring for ≈ 2 hr at room temperature. For epidermis, isolated from skin at 55°C (29), extractions were performed by heating in the above SDS - DTE - phosphate buffer solution for 5–10 min in a boiling water bath followed by vigorous vortexing. Solubilized protein was recovered by centrifugation between extractions. The slower rate of protein extraction from the nail plate refl ects the slower rate of pulveri- zation during magnetic stirring. Illustrated are the means and standard deviations of 4–6 samples of each type.
JOURNAL OF COSMETIC SCIENCE 232 phosphate (pH 7.8) - 20 mM DTE and then pulverized with a magnetic stirring bar for several hours. Aliquots of the solubilized and insoluble fractions were alkylated with iodo- acetamide, and the solubilized protein was recovered as a fl occulent precipitate by addi- tion of ethanol to 70%. The two fractions were rinsed in 70% ethanol and fresh 0.1 M ammonium bicarbonate and then digested with stabilized trypsin (≈1% by weight) in the bicarbonate buffer adjusted to 10% in acetonitrile. For this purpose, TPCK-treated bo- vine trypsin was stabilized by reductive methylation (17). Yields were improved by diges- tion for 2-3 days at room temperature with daily additions of trypsin. At the conclusion of digestion, the samples were clarifi ed, taken to dryness, submitted for mass spectromet- ric analysis and the data presented using Scaffold Proteomics Software (18). The reports permit ready access to many details of the protein identifi cation including degree of pep- tide coverage, spectral characteristics, unique peptides and statistical description. The original fi ndings from our analyses (19) were that the solubilized protein fraction of hair shaft was comprised of a score of keratin and keratin-associated proteins known to be present, while the insoluble fraction was much more complex. Using two chromatographic steps of peptide separation permitted identifi cation of some 350 proteins in the latter fraction originating from all regions of the cell. Careful examination of the peptide frag- mentation patterns permitted identifi cation of methylated lysines in a dozen proteins and evidence of ubiquitination as a glycylglycine posttranslational lysine modifi cation of ubiq- uitin itself. For these analyses, the peptide digest was fractionated by ion exchange chro- matography, and each fraction was then submitted to online reverse phase chromatography and mass spectrometry (LC-MS/MS). In the interest of higher throughput in later work, the fi rst step of ion exchange chromatography was omitted subsequently. Although not as comprehensive, the results typically identify 100 proteins in a given sample, including novel components, a benefi t of this discovery approach. Quantitating protein amounts in the shotgun approach is a challenge in general due to the unknown yields of peptides in mass spectrometry. Contributing further to the uncertainty are the possibly incomplete yields from digestion of cross-linked material and the inability to identify peptides derived from protein cross-linkage sites. Since only a minority of ly- sines are involved in isopeptide linkages, estimated as ≈18% for epidermal callus (20), suffi cient peptides are generated from the proteins to permit successful identifi cation. With these caveats, estimates have been made of relative amounts based on the empirical relation between the protein abundance and the number of unique peptides (i.e., not counting overlaps in sequence coverage) detected compared to those predicted (21). Although only semi-quantitative, estimates derived by this relation, called an exponen- tially modifi ed protein abundance index, offer a convenient way to rank proteins in relative abundance (22). APPLICATIONS OF SHOTGUN PROTEOMICS This approach to human hair syndromes exhibiting structural abnormalities plausibly will permit identifying protein defi ciencies, although it is not guaranteed to do so. In the case of a family displaying autosomal recessive woolly hair, samples from affl icted offspring did not show any defi ciencies in the prominent proteins identifi ed (23). In this instance, the lipase H gene was found to be defective by genetic testing. The availability of numerous mouse strains with anomalous structures suggests proteomic analysis could be useful in
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)