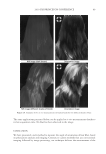
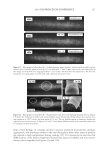
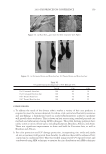
J. Cosmet. Sci., 62, 161–177 (March/April 2011) 161 Use of statistical modeling to predict the effect of formulation composition on coacervation, silicone deposition, and conditioning sensory performance of cationic cassia polymers CAROLE LEPILLEUR, JOHN MULLAY, CAROL KYER, PAM McCALISTER, and TED CLIFFORD, Lubrizol Advanced Materials, Inc., Noveon® Consumer Specialties, 9911 Brecksville Road, Brecksville, OH 44141. Synopsis Formulation composition has a dramatic infl uence on coacervate formation in conditioning shampoo. The purpose of this study is to correlate the amount of coacervate formation of novel cationic cassia polymers to the corresponding conditioning profi les on European brown hair using silicone deposition, cationic polymer deposition and sensory evaluation. A design of experiments was conducted by varying the levels of three surfactants (sodium lauryl ether sulfate, sodium lauryl sulfate, and cocamidopropyl betaine) in formulations containing cationic cassia polymers of different cationic charge density (1.7 and 3.0m Eq/g). The results show formulation composition dramatically affects physical properties, coacervation, silicone deposition, cationic polymer deposition and hair sensory attributes. Particularly, three parameters are of importance in determin- ing silicone deposition: polymer charge, surfactant (micelle) charge and total amount of surfactant (micelle aspect ratio). Both sensory panel testing and silicone deposition results can be predicted with a high confi - dence level using statistical models that incorporate these parameters. INTRODUCTION Common conditioning shampoos are formulated with cationic polymers such as cationic cellulose or cationic guar derivatives which are compatible in the shampoo formula, but become incompatible upon dilution with water. The literature suggests that upon sham- poo application, foaming, washing and rinsing, such cationic polymers form a complex with anionic and amphoteric surfactants that phase separates from the bulk solution, at surfactant concentrations below their critical micelle concentration (cmc). This phase separation or coacervation is known also as the “Lochhead effect” (1). The phase separa- tion upon dilution has been mainly explained in the literature through coulombic attrac- tion between anionic function of the surfactant and the cationic groups of the polymer. Goddard et al. (2) described that, at low surfactant concentration (below the cmc), anionic surfactants condense on the polycations. The resulting ion pair converts the cationic sites into hydrophobe-substituted sites. These hydrophobic interactions within and between
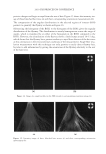
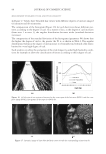
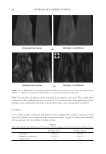
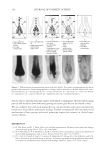
JOURNAL OF COSMETIC SCIENCE 162 the polycations cause a phase separation. The phase separation persists if the positive and negative charge equivalent ratio is at stoechiometry. Above the surfactant cmc, co-micel- lization of the cationic polymer with the surfactant results in a one-phase soluble complex or clear system. The coacervate is often described as a gel-like phase that contains a high level of cationic charge and is known to deposit the polymer on negatively charged hair, forming a clear fi lm (3-5). In addition, the coacervate aids in the deposition of insoluble actives such as silicone. The coacervation behavior and the type of coacervate formed vary depending on many criteria such as the cationic polymer characteristics (charge density and molecular weight), the cationic polymer concentration, the surfactant package used in the formulation, the ionic strength, pH and temperature. Li et al. (6) reported that the type or rheology of the coacervate formed infl uences the conditioning response. For in- stance, a highly charged cationic cellulose polymer may form a solid, granular, gel-like coacervate with low water content over a narrow dilution range that tends to be substan- tive and provide body to hair. A polymer with lower charge density may form a liquid- like gel with high water content over a wide dilution range that may be associated with soft feel and volume enhancement for hair. The molecular weights of these cationic poly- mers were shown to infl uence the amounts of coacervate, where the high molecular weight polymers formed more coacervate than the low molecular weight polymers. The objective of this study is to determine the effect of the surfactant formulation compo- sition on the conditioning performance of novel polymers based on cassia galactomannans. A design of experiments, as illustrated in Figure 1, considers the infl uence of the levels of three surfactants commonly used in shampoos sodium lauryl ether sulfate (SLES-3), sodium lauryl sulfate (SLS), and cocamidopropyl betaine (CAPB) in formulations containing differ- ent cationic cassia polymers. It is known that the formulation composition has a dramatic effect on physical properties (viscosity, clarity, turbidity) and also on the coacervation behav- ior (7,8). The purpose of this study is to try to correlate the amount of coacervate formation to the conditioning profi les on European brown hair through the deposition of a small par- ticle size silicone emulsion (average silicone droplet size of about 0.5 μm). Figure 1. Design of experiments for shampoo formulations using surfactant combinations containing various levels of SLES-3 (x axis), CAPB (y axis) and SLS (z axis) (12 formulations).
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)