J. Cosmet. Sci., 62, 229–236 (March/April 2011) 229 Proteomic analysis of hair shaft and nail plate ROBERT H. RICE, Department of Environmental Toxicology, University of California, Davis, CA 95616-8588. Synopsis The protein components of living cells in the hair follicle are amenable to study by standard molecular biolog- ical techniques, but identifying those in the hair shaft has been problematic until recently. Most of the protein, primarily keratins and keratin associated proteins, can be extracted under denaturing conditions, but 15-20% is intractable due to transglutaminase-mediated cross-linking. Shotgun proteomics now permits identifying 300 constituents of the isopeptide cross-linked proteome and even certain post-translational modifi cations. The proteins originate from all the intracellular compartments, indicating that the cross-linking process makes effective use of available resources to produce structures with great mechanical stability. Knowing this proteome provides a foundation for correlating defects in hair shaft structure with protein defi ciencies. Such investigations can be extended to mouse models of aberrant pelage hair. Thus, inbred mouse strains can be distinguished by their hair proteomes, raising the possibility of similar variation in the human population. The nail plate is also amenable to this shotgun proteomic approach. Providing discrete and noninvasive sam- pling of the human proteome, these epidermal appendages could have diagnostic utility for certain disease states. BACKGROUND Mature corneocytes in hair shaft, nail plate and epidermal callus are designed by nature to resist external physical stress and chemical exposures. They are comprised largely of kera- tin and keratin-associated proteins surrounded by an envelope of cross-linked protein. While the majority of protein is extractable from these corneocytes under strongly dena- turing conditions, a substantial fraction (15-20% in the case of hair) resists solubilization due to considerable transglutaminase-mediated isopeptide bonding. An inability to sepa- rate constituent proteins has prevented their identifi cation until very recently. Microscopy of the structures has revealed important physical features, and immunohistochemical studies of developing regions bordering the mature ones has permitted identifi cation of major components. The powerful approach of targeted gene ablation provides comple- mentary information on structural and developmental defects. Now that mass spectrome- try, coupled with database searching of peptide masses, permits identifi cation of proteolytic fragments of complex protein mixtures, the identities of the components of the cross- linked material are now being revealed. Studies by pioneering dermatologists a half century ago pointed to a particularly resistant structure at the outer boundary of corneocytes in the callus layer of the skin (1,2). Similar features are seen in the nail plate, where the interlocking borders of adjacent cells provide
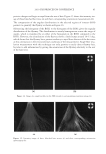
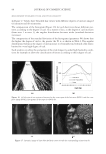
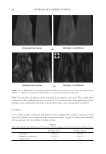
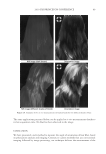
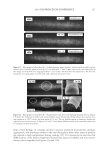
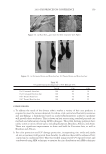
JOURNAL OF COSMETIC SCIENCE 230 great cohesiveness to the overall structure (3). The native hair shaft presents diffi culties in transmission electron microscopy due to poor penetration by embedding media without treatment to permit its diffusion into cellular interiors. A simple such treatment (Figure 1) is to incubate the hair in sodium dodecyl sulfate (SDS) under reducing conditions for an hour or two at room temperature, inducing swelling and softening of the fi ber and permitting ultrastructural visualization of bundles of intermediate fi laments in the cortex (4). More extensive detergent extraction at elevated temperature yields nearly empty cor- tical cells with their outer boundaries clearly delineated. By contrast, cuticle cells appear largely intact, with clear demarcation of the endocuticle, exocuticle and marginal band. Cells of the medulla contain remnant nuclei and amorphous deposits as well as areas with- out electron dense material. The cross-linking process in hair resembles that in the analo- gous cornifi ed appendages of bird feather and hagfi sh teeth, and thus occurs generally among vertebrates (5). Defects in the cross-linking process can be visualized microscopically in samples from epi- dermis and appendages after extraction of solubilizable protein with SDS and reducing agent. Individuals defi cient in this process can exhibit lamellar ichthyosis and related skin conditions such as congenital ichthyosiform erythroderma characterized by high rates of transepidermal water loss and prominent scaling. Instead of displaying the normally ob- served empty cells with distinct boundaries, samples of extracted epidermal scale appear fragmented and disorganized. Small pieces of nail plate show analogous alterations in the diseased state with loss of the normally obvious cell borders (6,7), while the hair cuticle shows considerably increased extraction or loss of features such as the marginal band (8,9). Structural alterations in the cortex and cuticle of uncertain basis are also seen in hair from individuals affl icted with trichothiodystrophy (8) and in the cuticle from mouse mutants such as matted and naked (10). Based on original work on wool and hair in animal models, trapping of protein constitu- ents in insoluble complexes was attributed to the action of transglutaminases found in the hair follicle (11,12), analogous to the action of factor Xllla in blood clotting (13). Studying Figure 1. Swelling of hair fi bers at room temperature. Fibers from a Caucasian male were incubated in water or 0.1 M sodium phosphate (pH 7.8), in the latter case with 20 mM DTE and/or 2% SDS. Samples were ex- amined after 6 hr except as indicated (2 hr). Shown are the means and standard deviations of diameters of 6–30 fi bers for each condition measured microscopically (16×) using Slidebook 5.0 software. Swelling and increased fl exibility are fi rst noticeable visually after 1–2 hr, at which time hair fi bers can be fi xed and embedded.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)