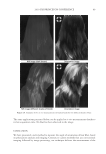
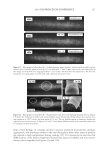
2010 TRI/PRINCETON CONFERENCE 181 are shown in Figure 1 above. Polyquaternium-7 and -10 were used because of their known coacervate formation and use in personal care products. Polyquaternium-76 and -88 are two relatively new polymers that are slated for this application. Two of the most common surfactants used in body care in combination with the cationic polymers are sodium lauryl sulfate (SLS) and sodium laureth sulfate (SLES) (Figure 2). Sodium laureth sulfate is commercially available in a range of degrees of ethoxylation. The most commonly used variants have degrees of ethoxylation ranging from one to three. Many co-surfactants have also been used in commercial shampoos, principally cocobetaines and distearates. However, this study was directed only to the interaction with the primary anionic surfactants. Our experiments were limited to SLES with two ethyl- ene oxide groups. This molecule is abbreviated as SLE2S or SLES-2EO. POLYMER/SURFACTANT INTERACTIONS The aggregation process of coacervate formation between oppositely charged polyelectro- lytes and surfactants with has been explained by Dubin and coworkers (3) as electrostatic interactions between the oppositely charged polymer molecules and surfactant micelles in which there are two distinct regions of coacervate formation at different micelle charge densities. The Dubin model indicates that micelle charge density controls coacervate formation. For oppositely charged polyelectrolyte and surfactant molecules, the surfac- tant binds to the polyelectrolyte due to electrostatic attractive forces. As the complex neutralizes, coacervation occurs as the intrapolymer complexes develop into interpolymer aggregates. At higher micelle charge density, coacervate precipitation occurs due to strong electrostatic interactions. Polyquaternium-7 and Polyquaternium-10, have been used in formulations over the last 30 years. They have large differences in charge density4. According to the Dubin model, these differences of charge densities explain the different interactions these polymers have with surfactants in a system. On the other hand, Goddard et al. (5–7) have proposed that the coacervate formation process is a site-specifi c ion-ion interaction. Here, the cooperative process between the electrostatic interaction and hydrophobic association/segregation governs complex formation. The asso- ciation between the surfactant anion and the polymer cations is driven by the resultant loss of free energy. Thus, a loss of enthalpy accrues from the ionic attraction between polyions and surfactant ions of opposite charge, and the release of soluble counter-ions into the sur- rounding media results in a gain in confi gurational entropy. The formation process is also driven by hydrophobic interaction between tail groups of the bound surfactant molecules, resulting in intramolecular and intermolecular association of these groups. Goddard et al. (5,7) and other researchers (8,9) observed that complexes formed be- tween the oppositely charged polyelectrolyte and surfactant system depends on the surfactant concentration and involves surfactant binding, phase separation and re- solubilization. Figure 2. Structures of sodium lauryl sulfate and sodium laureth sulfate.
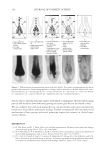
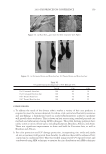
JOURNAL OF COSMETIC SCIENCE 182 Figure 3. Schematic of surfactant alignment during foam formation. Goddard (7) and other researchers (10,11) demonstrated that above the critical concentra- tion of the surfactant, known as the critical aggregation concentration (CAC), of the surfactant site-specifi c interactions occur between anionic surfactant molecules and cat- ionic sites along the polymer backbone. Increased interactions between the polymer and surfactant molecules are driven by increasing the surfactant concentration. However, a threshold occurs where the polymer-surfactant complex phase separates from the aqueous solution to form a polymer-surfactant coacervate. As the concentration of the surfactant is increased above the critical micelle concentration (CMC) of the polymer-surfactant system, the coacervate can become soluble to form a single phase system. The basic, yet unresolved, difference between Goddard’s hypothesis and Dubin’s hypo- thesis derives from Goddard’s explanation that coacervation results from single ion-ion interaction whereas Dubin’s explains the phenomenon as arising from a colloidal interac- tion between the polyelectrolyte and the surfactant micelles. COACERVATE IN THE FOAM Upon foam formation (Figure 3), surfactant molecules adsorb at the air–water interface of the foam fi lm. As discussed above, at certain concentrations of polymer and surfactant, coacervates form in the solution. As foaming/lather formation occurs, it has been explained that the coacer- vate present in the solution is adsorbed into the crust of the foam lamella as shown in Figure 4. The high viscosity coacervate gel is present in the lamella crust, while the low viscosity phase is present in the lamellar core (12).
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)