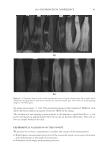
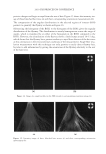
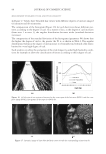
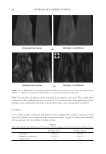
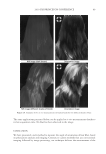
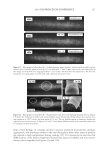
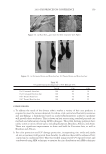
2010 TRI/PRINCETON CONFERENCE 123 melanocyte precursors in the stem cell niche in mice (4). In addition, gray hair follicles have almost absent catalase and methionine sulfoxide reductase expression (5) and are hence not able to fi ght oxidative stress dependent deterioration of hair follicle melanocytes. Establishment of a pigmentary unit requires various biological mechanisms. Melanocyte precursors (melanoblasts) must be recruited from the bulge which is the stem cell niche in the hair follicle (4,6). From there they must migrate down the outer root sheath (ORS) to the hair bulb and home at the tip of the dermal papilla above the Auber’s line where they form the pigmentary unit. Melanoblasts must differentiate into mature melano- cytes, synthesize melanin, the pigment that gives the hair its color, and pack the melanin into melanosomes. The melanosomes travel out to the tips of the melanocyte’s dendrites where they are transferred to keratinocytes. This complex mechanism is steered by gene expression and factors regulating hair follicle melanocyte biology. Silencing of the receptor for stem cell factor c-kit was shown to block establishment of a pigmentary unit in hair follicles (7,8). This shows nicely that hair fol- licle melanocytes are dependent on the growth factors and cytokines comprising the hair follicle growth milieu secreted by the epidermis and dermis surrounding the hair follicle. This growth milieu is changed constantly by intrinsic and extrinsic stimuli, and we know that during aging this milieu changes from a growth milieu into an aging milieu (9). Since melanocytes are cells derived from the neural crest, neurotrophic factors like nerve growth factor or neurotrophin 3 can regulate melanocytes development (10). In addition, Substance P, a neuropeptide stress mediator, has been shown to be present in nerve fi bers in close vicinity to stressed hair follicles, leading to apoptosis and disappearance of melanocytes from the pigmentary unit (11). In vitro we can study and assess the pigmentation status of individual hair follicles, iso- lated from scalp biopsies, by light microscopy and the so called likert-Scale. With the Likert-Scale we can distinguish fi ve grades of pigmentation from fully pigmented to white. These are assessed by defi ning the pigmentation status of the hair follicle’s pig- mentary unit (Figure 2). In fully pigmented hairs the pigmentary unit is a clear-cut pear- shaped black structure at the tip of the dermal papilla above the Auber’s line. During graying melanocytes are lost, the shape of the pigmentary unit gets fuzzy, melanocytes are dislocated and appear below the Auber’s line, it is possible to see the dendritic shape of the melanocytes and the dermal papilla becomes more and more visible. In white hair follicles fi nally there are no melanocytes left in the hair blub indicating the exhaustion of the melanocyte cell pool. Methods to study the graying process and results that can be obtained through their application. Two questions arise: why is there exhaustion of the regenerative capacity of hair follicle melanocytes, and how does the surrounding growth factor milieu change with age? To answer these questions and to better understand graying one would have to further char- acterize the molecular factors defi ning aging of melanocytes in the hair follicle. We spec- ulate that within the hair follicle gene expression is altered in white and gray hair follicles compared to pigmented hair follicies. Investigation of differential gene expression should provide a base to understand the genetic determination of hair follicle aging (12). In addi- tion, one could in vitro analyze the aging milieu in the hair follicle, e.g. through secreted factors (cytokines, neuropeptides, growth factors) in the growth medium or through in loco immunohistochemistry of pigmented versus gray or white hair follicles. One would
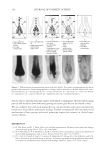
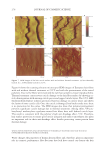
JOURNAL OF COSMETIC SCIENCE 124 then be able to identify molecular targets with which to manipulate the hair follicle aging process and therefore slow down hair graying or restore gray hair to its natural colour. We are confi dent that with such approaches one can specifi cally target genes and proteins involved in hair follicle melanocyte biology. Further investigation will elucidate novel mechanisms of hair graying and reveal promising targets for cosmetic or clinical inter- vention therein. REFERENCES (1) D. Van Neste and D. J. Tobin, Hair cycle and hair pigmentation: Dynamic interactions and changes associated with aging, Micron, 35(3), 193–200 (2004). (2) N. V. Botchkareva, V. A. Botchkarev, and B. A. Gilchrest, Fate of melanocytes during development of the hair follicle pigmentary unit, J. Invest. Dermatol. Symp. Proc., 8(1), 76–79 (2003). (3) P. C. Arck, R. Overall, K. Spatz, C. Liezman, B. Handjiski, B. F. Klapp, M. A. Birch-Machin, and E. M. Peters, Towards a “free radical theory of graying”: melanocyte apoptosis in the aging human hair follicle is an indicator of oxidative stress induced tissue damage, FASEB J., 20(9), 1567–1569, (2006). Figure 2. Differentiation of pigmentation status in the hair follicle. Five grades of pigmentation are distin- guished and assessed by characterizing melanocyte density and localization in the hair follicle bulb. bm – basal membrane dp – dermal papilla em – ectopic melanocyte hs – hair shaft irs – inner root sheath m – melanocyte ors – outer root sheath pu – pigmentary unit rm – rounded melanocyte.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)