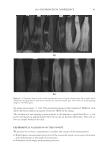
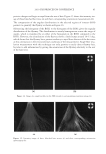
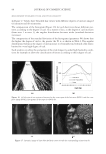
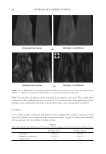
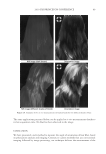
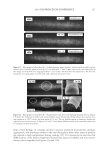
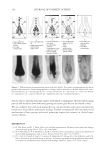
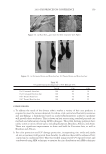
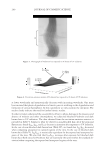
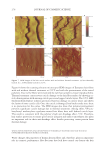
2010 TRI/PRINCETON CONFERENCE 119 with low hot-iron speeds. Instead the polymer shifted their formation temperatures to levels higher than 220°C. In summary, the above described experiments suggest that the following mechanism is responsible for heat damage to the cortex in hair fi bers subjected to hot irons. When a hot iron is in contact with hair heat transfer takes place. This heat transfer creates a temperature gradient inside a hair fi ber from the cuticle sheath to the center of the cortex. The profi le of this temperature gradient is dependent on the contact time of the hot iron with the hair fi ber. If the hot-iron speed is fast or normal the gradient will be steep, and only regions at the periphery of the hair cortex will get affected by heat fl ow and high temperatures. Thus, protein denaturation and explosive water evaporation will occur mainly at the cortex periphery leading to the production of micropores in these regions such as observed with normal hot iron speeds and temperatures ≤ 180°C (see Figures 2 and 7). In contrast, when the hot-iron speeds are reduced, a larger contact time between hot-iron and hair surface is established. This will allow for a higher heat transfer and ultimately will lead to higher temperatures inside the hair fi ber causing, thereby, an incipient pro- cess of protein melting and shrinkage. Consequently, low hot iron speeds and tempera- tures between 180°C T ≤ 210°C will produce larger voids deeper inside the cortex (see Figs. 10 and 11). For hot iron temperatures higher than 220°C at low speeds more heat fl ow will take place. This process will generate high temperatures inside the hair with values very close to those at the hot-iron surface. These high temperatures will create, thus, the massive process of protein melting, shrinking, and aggregation with the conse- quent production of large bubbles, hair shape distortion, and fi ber fragmentation as de- picted in Figs. 12 to 13. Finally, the effect of polymer layers on the formation of micropores can be explained by considering that, when a polymer material is deposited on the hair surface, it acts as a thermal buffer that slows heat transfer and reduces the explosive water evaporation that occurs in regions deep inside the cortex. It is worth mentioning here, that the presence of thin layers of polymers on the hair surface also modifi ed the patterns of friction damage at the cuticle sheath. The topic of friction damage by hot-irons is presently being studied and will be the subject of another report. CONCLUSIONS Heat damage by hot irons was found to be dependent on the heat transfer to hair and to be determined by the conditions of temperature and hot-iron speed. The various types of damage appearing in the hair cortex in the form of micropores, large voids, and air bub- bles were found to involve protein changes ranging from protein denaturation, melting, aggregation, and shrinkage depending on the temperature. Water evaporation and mechanical extension were shown to play a critical role in the degree of micropore forma- tion. With normal hot-iron speeds and temperatures not higher than 200 °C most of the damage in the form of pores was confi ned to the cortex periphery and to the cuticle sheath. With higher temperatures and lower speeds damage was extended to regions deeper inside the cortex. The presence of polymer layers on the hair surface appears to shift micropore production from the cortex to the cuticle sheath and also, to increase the resistance for the formation of larger voids.
JOURNAL OF COSMETIC SCIENCE 120 REFERENCES (1) P. Milczarek, M. Zielinski, and M. L. Garcia, The mechanism and stability of thermal transition in hair keratin, Colloid Polym. Sci., 270(11), 1106–1115. (2) J. Cao and F. Leroy, Depression of the melting temperature by moisture of the α-form crystallites in human hair keratin, Biopolymers, 77(1), 38–43 (2005). (3) F. J. Wortmann, G. Sendelbach, and C. Popescu, Fundamental DSC investigations of α-keratinous materials as basis for the interpretation of specifi c effects of chemical, cosmetic treatments on human hair, J. Cosmet. Sci, 58(4), 311–317 (2007). (4) D. Istrate, C. Popescu, and M. Moller, Non-isothermal kinetics of hard alpha-keratin thermal denatur- ation, Macromol. Biosci. 9(8), 805–812 (2009). (5) R. McMullen and J. Jachowicz, Thermal degradation of hair. I. Effect of curling irons, J. Cosmet. Sci., 49, 223–244 (1998). (6) Y. Zhou et al. Investigation of thermal damage of hair from hot fl at ironing and the thermal protective effects of cosmetic pretreatments, Proceedings of the 26th IFSCC Congress, Argentina, September 20–23, 2010. (7) S. B. Ruetsch and Y. K. Kamath, Effect of thermal treatment with a curling iron on hair fi ber, J. Cosmet. Sci., 55, 13–27 (1998). (8) R. L. McMullen and S. P. Kelty, Investigation of human hair fi bers using lateral force microscopy, Scan- ning, 23(5), 337–345 (2001). (9) C. Scanavez, M. Silveira, and I. Joekes, Human hair: Color changes caused by daily care damages on ultra-structure, Colloids Surf. B, 28(1), 39–52 (2003). (10) M. Schaffer, V. Hill, and T. Cairns, Hair analysis for cocaine: The requirement for effective wash proce- dures and effects of drug concentration and hair porosity in contamination and decontamination, J. Anal. Toxicol., 29(5), 319–326 (2005). (11) M. Feughelman, Mechanical Properties and Structure of Alpha-Keratin Fibres Wool, Human Hair, and Related Fibers (University of New South Wales Press, 1997). (12) S. Ogawa, K. Fujii, K. Kaneyame, K. Arai, and K. Joko, A curing method for permanent hair straight- ening using thioglycolic and di-thioglycolic acids, J. Cosmet. Sci., 51, 379–399 (2000). (13) L. J. Wolfram and M. K. Lindemann, Some observations in the hair cuticle, J. Soc. Cosmet. Chem., 22, 839–850 (1971). (14) J. Cao and A. Y. Bhoyro, Structural characterization of wool by thermal mechanical analysis of yarns, Textile Res. J., 71(1), 63–66 (2001). (15) T. R. Dutson and M. W. Orcutt, Chemical changes in proteins produced by thermal processing, J. Chem. Educ., 61(4), 203 (1984). (16) N-vinylpyrrolidone methacrylamide and N-vinyl imidazole BASF polymer Luviset Clear.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)