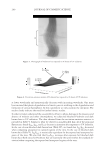
2010 TRI/PRINCETON CONFERENCE 129 of the surface tension, and the other liquid probe should have both the dispersive and polar components of the surface tension, such as water (σLP = 46.4 mN/m, σLD = 26.4 mN/m), or benzyl alcohol (σLP = 11.4 mN/m, σLD = 28.6 mN/m). The contact angle values measured from diiodomethane are used to calculate the dispersive component of the solid surface energy, and the contact angle values measured from water or benzyl al- cohol are applied to calculate the polar component of the surface energy (2,6,7). In previously published articles (2,6), surface energies of common polymer solids were calculated using the theories mentioned above and the surface energy data of different types of hair were also cited in the literature (9). In addition, the contact angle values of hair fi bers have been used to validate the hydrophobic/hydrophilic change of the hair before and after cosmetic treatment (10). Professor Bhushan and his colleagues have published several papers to explore nanotribo- logical characterization of hair (11,12), surface energy data of different hairs and changes in surface energy of hair fi bers after different treatments were also circulated (8). But till now, there is no publication studying correlations between changes in the micro-scale property of hair-surface energy and changes in the macro-scale property of the hair tresses combing force. In this paper, a method of determining the surface energy of hair fi ber samples is revealed. The changes in average surface energy of hair fi bers before and after conditioner treatment were correlated to the changes in combing forces of corresponding tresses. Experimental results verifi ed that a decrease in hair surface energy can be used to evaluate or screen the performance of cosmetic ingredients and formulations. EXPERIMENTAL MATERIALS AND INSTRUMENTS K • RŰSS Processor - Tensiometer K100MK2, from KRŰSS, USA Laser Scanning Micrometer, Mitutoyo LSM-5000, from Mitutoyo, Japan • Miniature Tensile Tester MTT-175 and MTT-160, from Dia-Stron Instruments, UK • Virgin brown and regular bleached hair were purchased from International Hair • Importers, Inc., New York. Quaternium-91 (and) cetrimonium methosulfate (and) cetearyl alcohol (Trade name: • CrodazosoftTM DBQ, Croda Inc., Edison, NJ) Behentrimonium methosulfate (and) cetyl alcohol (and) butylene glycol (Trade name: • IncroquatTM Behenyl TMS-50, Croda Inc., Edison, NJ) Conditioner-A contains 6.00% of Quaternium-91 (and) cetrimonium methosulfate (and) • cetearyl alcohol, 0.80% of Neolone CAPG, and the rest was balanced with DI-water. Conditioner-B contains 6.00% of behentrimonium methosulfate (and) cetyl alcohol (and) • butylene glycol, 0.10% of Neolone 950, and the rest was made up with DI-water. CONTACT ANGLE MEASUREMENT Virgin brown and regular bleached hair tresses were prewashed with 10% sodium • lauryl sulphate. Thirty hair fi bers were randomly selected, crimped onto two brass tabs, and the central • diameter of the hair fi ber was measured using a laser scanning micrometer.
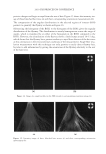
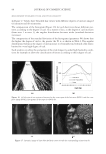
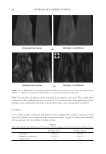
JOURNAL OF COSMETIC SCIENCE 130 Cut the hair fi ber into two parts: A and B. Part A was used as control without any • treatment, and part B was treated with testing conditioners. Dynamic advancing contact angle of single hair fi ber was measured using a KR • ŰSS Processor - Tensiometer K100MK2. The value of a determined contact angle in diiodomethane was used to calculate the dispersive component of the surface energy, and the measured value of contact angle in benzyl alcohol was used to calculate the polar component of the surface energy. The total surface energy of the hair fi ber is the sum of the dispersive and polar • components. COMBING FORCE MEASUREMENT Hair tress width is 1.2 cm, and the length is 20 cm. Virgin Brown and Regular Bleached • hair tresses were prewashed with 10% sodium lauryl sulphate (SLS) before use. Apply 2 ml of 10% SLS solution to the hair tress and manage it gently for 1 minute. • Work the tress from the top to the bottom to avoid tangling. Rinse the tress with running tap water for 1 minute. Let the tap water run down the • swatch from the top to the bottom to avoid tangling. For each formulation, fi ve hair tresses were used for combing force measurements before • and after respective treatment. Wet combing forces were determined at room temperature and dry combing force • measurements were performed in a chamber with a constant humidity level of 65%. Percentage changes in dry or wet combing forces of the same tress before and after respec- tive treatment were calculated and averaged for fi ve hair tresses. RESULTS AND DISCUSSION CHANGE IN AVERAGE CONTACT ANGLE VALUES Contact angle values of hair samples treated with conditioner-A were summarized in Figures 1 and 2, respectively, for bleached and virgin hair. Contact angle values of condi- tioner-B treated hair were illustrated in Figures 3 and 4, respectively. The lower bars represent contact angle values of the control hair, the taller bars show contact angle values of the treated hair samples. Two bars on the left side of each fi gure indicate contact angle values measured at the diiodomethane/air interface, and the two bars on the right side represent the contact angle values measured at the air/benzyl alcohol interface. It can be seen that average contact angle values of conditioner-A treated bleached hair fi bers increased by 25.07% in diiodomethane, and 21.97% in benzyl alcohol, respec- tively. While the average contact angle values of conditioner-A treated virgin hair in- creased by 12.54% in diiodomethane, and 25.04% in benzyl alcohol, respectively. It is clear that hair surface became more hydrophobic in both liquid probes in comparsion to the control samples without any treatment. As shown in Figures 3 and 4, the surface of hair samples treated with conditioner-B also became more hydrophobic. Average contact angle values of conditioner-B treated bleached hair raised by 25.54% in diiodomethane and 29.64% in benzyl alcohol, respectively.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)