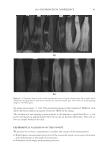
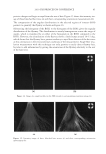
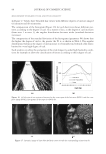
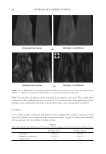
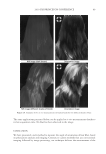
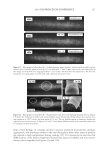
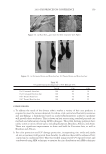
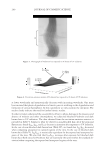
J. Cosmet. Sci., 62, 127–137 (March/April 2011) 127 Study of hair surface energy and conditioning TIMOTHY GAO, YINGXIA HE, PETER LANDA, and JUNG-MEI TIEN, Croda Inc., 300-A Columbus Circle, Edison, NJ 08837. Synopsis A new test method has been developed to determine surface energy of hair fi bers through measurements of contact angles at two hair/liquid interfaces. By measuring changes in surface energy of the same hair fi ber before and after a cosmetic treatment, effects of active ingredients and the performance of tested formulations can be evaluated. The establishment of the method is based on Fowkes theory (1,2) described with two components, a disper- sive and a non-dispersive component. The non-polar liquid used in this study was diiodomethane, and the polar liquid was benzyl alcohol. A Kruss 100 Tensiometer was used to measure contact angles of hair fi bers. Virgin dark brown and regular bleached hairs were treated with selected conditioner formulations. Reduc- tions in combing forces of hair tresses before and after respective treatments were correlated with decreases in average surface energy of hair fi bers obtained from the corresponding tresses. Experimental results indicate that the average surface energy of hair fi bers treated with conditioners decreases and the hydrophobicity of the hair surface increases, the results correlate well with the reduction in combing forces after respective treatments. This research work provides a new methodology to evaluate/screen condi- tioning performance of hair care ingredients and formulations for development of better products. INTRODUCTION Surface energy is one of important physico-chemical properties of solid materials, as it impacts surface properties of the solid, such as wetting, spreading, and adhesive perfor- mance. Surface energy of hair affects how cosmetic treatments interact with hair surface, it can be used to judge the nature of the hair, as healthy hair always has lower surface energy compared to that of damaged hair. All cosmetic products applied onto hair surface actually change the surface energy of the hair. In order to improve hair conditioning per- formance, formulations should modify the treated hair surface and make it more hydro- phobic with lower surface energy. Researchers from Textile Research Institute in Princeton have published several papers on studies of changes in wettability of keratin fi bers after different treatments (3–5). The surface wettability or contact angle at the fi ber/water in- terface is related to the surface energy, but not equal to that. Therefore, it is important to study changes in surface energy of hair fi bers after different treatments. Though measuring the surface energy of a liquid is simple and direct, because the surface energy of a liquid equals the surface tension of the liquid, but determining the surface energy of a solid is not as straightforward (2). There are several published articles that
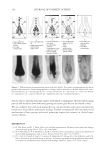
JOURNAL OF COSMETIC SCIENCE 128 describe the calculation of polymer surface energy through the measurement of the con- tact angle, by applying theories such as the Zisman theory, the Owens/Wendt theory, the Fowkes theory, and the Van Oss theory (2,6,7). Mr. Brown and his co-workers from Procter & Gamble Company disclosed a method to measure surface energy of hair fi ber by using an image device (8). They measured contact angles of hair fi ber in two solvents (99%+ pure hexadecane and ultrapure water) and calculated surface energy according to Fowkes equations. The Fowkes theory is a combinaton of three equations that describe the interfacial inter- actions between the liquid and the solid (2,6). The fi rst equation is Young’s equation: s = s + sL cosq S SL (1) where σs = total surface energy of the solid, σSL = the interfacial tension between the liquid and the solid, σL = the surface tension of the liquid, and θ = the contact angle at the liquid/ solid interface. Another important equation used in the Fowkes theory is Dupre's adhesion energy: = s + s - sSL SL S L I (2) where ISL is the adhesive energy between the liquid and the solid surface. The Fowkes theory separates the adhesive energy into the dispersive component which is attributed to the non-polar interaction of the interface, and the polar component that is contributed by the polar interaction at the liquid and solid interface. Therefore, the ad- hesive energy equation (2) becomes equation (3): ( ) ( )1/2 ª º = s s + s s « » 1/2 2 D D P SL L S L S I (3) in which the interfacial tension variable is eliminated from the equation, and the combi- nation of equations (1), (2), and (3) yields the Fowkes equation (4): ( ) ( )1/2 ( )/2 q s s + s s = s +1 1/2 cos D D P L S L S L (4) in which, s D L = the dispersive component of the surface tension of the liquid, s D S = the dispersive component of the surface energy of the solid, sLP = the polar component of the surface tension of the liquid, s P S = the polar component of the surface energy of the solid, the total surface energy (σS) of a solid material is the sum of the dispersive surface energy (s D S ), and the polar surface energy (s P S ), is as described in equation (5): s = s + sS D P S S (5) To use the Fowkes equation to calculate the surface energy of a solid surface, the contact angle of the solid needs to be measured in tow liquid probes. One recommended liquid probe is Diiodomethane (σL = σLD = 50.8 mN/m), which has only dispersive component
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)