298 JOURNAL OF COSMETIC SCIENCE customers with analyses of hair fibers using sophisticated techniques that are accurate methods per se. The basis for an analysis of such data remains an uncertain one as yet (cf. refs. 9-11). From the biologist's/physician's point of view there are two main objections to such analyses. The first concerns the manner in which hair is collected for analysis. Generally this has not taken the state of the hair cycle or the scalp area of origin into consideration i.e., the analyses are made on bulk specimens rather than on selected hair fibers in the anagen (growing) phase of the hair cycle. If the analysis data are to be related to the physiologic history of the proband, such as is the case in lawsuits and forensic applica- tions, the timing of ingestion of (a) specific element(s) can be deduced from the distance to the hair root, since hair growth in normal individuals is relatively constant (approxi- mately 0.4 ram/day). Using bulk specimens would mean that we lose this kind of information. The increased mass of the specimen will not speed up the analysis process, which is a matter of a few minutes with single fibers, and adds the problem of correction for self absorption, which is virtually nil with single-fiber analysis. The second is that there is presently no reference material publicly available for comparison of individual data. In addition, ideally such quantitative elemental data from hair fibers should be correlated to quantitative data from blood analyses of the same individuals. Furthermore, there is a need for the establishment of precision and accuracy in single-fiber measure- ments. These topics have been scrutinized in the past (5,14), but the effect of such analyses and discussions does not seem to have influenced the commercially interested parties. In forensic applications, hair fibers collected during crime investigations are often ex- amined for morphological characteristics, and sometimes also for cosmetic treatments, chemical analysis of shampoo residues (1), and DNA or enzyme analysis. In some instances such analyses allow a specific hair fiber to be assigned to a certain individual. However, only in a minor number of cases are such characteristics distinctive enough to be used as conclusive evidence. With the development of new DNA techniques, hair fibers can sometimes be identified as to their origin by comparative analysis, being a reason for replacement of enzymatic tests. Elemental analysis has not yet proven to be useful for identification of single hair fibers, but as a method of analysis complementary to that of DNA techniques, it may provide information on subjects related to physi- ological status, residential area (rural/urban), certain occupations (e.g., uptake of ele- ments at a particular time), etc., of specific individuals. A basis for such evaluations must rely on good baseline data of the normal elemental content in hair fibers from a normal population. The present study makes it clear that, when the goal is to assess an individual's physi- ological status, elemental analysis of hair should be made on a virgin part of a single anagen hair fiber, i.e., within the segment 2-5 mm from the root of a plucked hair fiber in order to minimize effects of contamination or leakage out of the hair shaft. However, the question of how representative such a measurement is, as related to a database of normal values that is thought to represent a large population, remains a crucial one. A previous investigation suggested that even the hair fibers from one and the same location in an individual might show conspicuous variations in the concentration of elements such as sulfur (S) and zinc (Zn) (9). Many analytical techniques are essentially bulk techniques requiring at least milligram
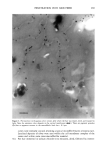
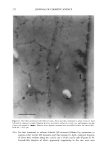
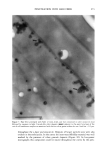
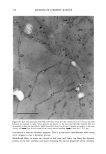
ELEMENTAL DISTRIBUTION IN HAIR 299 amounts of hair, making them practically useless for analysis of virgin parts of hair. In forensic applications and studies of certain medical disorders, such amounts are not available and single-fiber analysis becomes necessary. This type of analysis has until now only been possible with particle probes and synchrotron light (2,9,10,18,22-24), which allows the analysis of very small volumes, making it possible to reanalyze the same part of the specimen. We will demonstrate that the X-ray fluorescence (XRF) technique presented here has the advantage of being highly sensitive at trace-element levels of detection and that, in addition, it is a non-destructive technique. In the present study we have investigated triplets of anagen (actively growing) hair fibers collected from the temporal area of 63 normal Caucasians, using XRF analysis in a new energy-dispersive analysis system. It was not possible to obtain a blood sample simul- taneously with the collection of hair fibers. MATERIALS AND METHODS THE INSTRUMENT ITRAX •, is a new commercially available X-ray spectrometer used for the analyses (Figure 1). The ITRAX system is useful for bulk and trace element analysis in small samples, using X-Ray fluorescence (XRF) analysis. The detection limits depend on the photon energy emitted from the excited sample by a particular element and reach down to single tag/g for some elements in hair (Figure 2). The principles of the instrument have been previously described (6,7,15,16,21). Briefly, the X-ray source, which can be op- erated at a maximum of 60kV/50mA, is an X-ray tube with a molybdenum (Mo) anode. The X-rays are transmitted to the sample through a conical glass capillary that has diameters of 0.5 mm at the end facing the anode and 0.3 mm at the outer end, allowing for a bremsstrahlung reduction and an intensity increase by concentration of the X-rays on the specimen through subsequent multiple reflections inside the capillary. The divergency of the beam at the exit is less than 0.2 ø . The measuring area of the instrument is protected by a plastic hood, and it is operated in air. In the particular setup for hair analysis, the position of the sample can be visualized through two CCD cameras equipped with lenses focused on the object. One camera faces the sample in a direction opposite to the extension of the impinging X-ray beam the other camera operates from above. These cameras are connected to a video monitor which also allows measurement of the fiber cross section seen by the camera as well as the position of the beam seen by using a fluorescent screen placed in the beam. The fluorescent and scattered X-rays were registered using a 30-mm 2 energy-dispersive Si(Li) detector with a Link 2040 pulse processor placed at a 90 ø angle to the beam pulse processor. The detector of the specific equipment used has 146 eV resolution at 5.9 keV and is connected to a Camberra-Packard multi-channel analyzer installed in a Win- dowsT•4-based PC. Spectra were evaluated using a spectrum evaluation program (Spetest, Cox Analytical) Cox Analytical Systems AB, Teatergatan 36, 411 35 Gothenburg, Sweden.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)