308 JOURNAL OF COSMETIC SCIENCE concentration will be influenced by the degree of re-distribution, extraction, and/or contamination from the environment, cosmetic and hygienic effects included. In our study such variations appear to lie within expected biological variation limits for Cu, whereas K increases conspicuously above a position approximately 2 mm from the root (position 10), further emphasizing the necessity that hair analyses shall be confined to the virgin part of the hair fiber. A similar distribution pattern is also evident for Ca, which is an expected contaminant through its general presence in (tap) water. The stability of the concentration of Cu gives further support to the idea that Cu and Zn are better choices for internal standard reference than S in semi-quantitative analyses (11). However, tap water and pool water may contain appreciable amounts of Cu 2+, which may bind strongly to the hair fiber, especially if the water is chlorinated (22). The best candidate for an internal standard is therefore Zn. In this work, an analytical time of twelve minutes was chosen for each hair. This was a reasonable compromise between efficiency of work and precision in the measurement of elements present at low concentrations. It is difficult to make sure that a hair fiber is completely free from contaminants on the surface. To be reasonably confident, reanalyses of all measurements, with cleanings in between, should be performed for a comparison of the results. Such measurements are beyond the scope of the present investigation. However, reproducibility tests and the performed reanalyses of hairs with high levels of some elements show that the problem is not a severe one, albeit existing. In addition to the endogenous variation in elemental content expected from a biological sample, there are effects of measurement variations due to contamination and the intrinsic variability of the physical measurement system. In hair analysis the contamination appears to be the most serious problem. To minimize the effects of extreme data values, we have used the empirical median value for each individual (three or six hairs) in computation of elemental content (Table II). No significant discrepancies between the washed and unwashed hairs were observed. Ex- treme values in some fibers are especially obvious in the cases of K and Ca, elements that can be redistributed and extracted by contact with water. Ordinary tap water used for hygienic and cosmetic purposes, in addition, may contaminate the hair. However, with three or six independent measurements (corresponding to the number of analyzed hair fibers from each person), incorrect data from single measurements will not affect the calculated median values for each person (Table II). Calculation of concentrations has been made based on the assumption of a hair diameter of 0.045 mm. An actual diameter below this value results in an overestimation when calculating the concentrations, especially for the lighter elements like S and C1. In fact, the diameter will have strong influence on the measurement of these elements due to the attenuation the characteristic X-rays undergo in the hair bulk material (9). For higher energy characteristic lines, and for the intensity of scattered radiation, this attenuation effect becomes negligible as compared to other variations of biological material. Since the actual diameter of each hair fiber has not been measured, a discrepancy in measured data compared to the true values is likely to exist. Furthermore, the calculations assume an even distribution over the cross section, which is not true for many of the elements (2,6,15), and this will also affect the results to some extent, again especially for light elements (18). Reproducibility is of crucial importance in hair analysis for diagnosis of illness or in
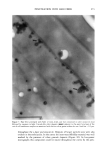
ELEMENTAL DISTRIBUTION IN HAIR 309 forensic examinations. In the present study a variation of the concentrations for elements in all hairs was recorded. To study the reproducibility of our method we scanned along a hair with high K content (Figure 4). As can be seen, the two scans correspond well for K and Ca variations, while as for Cu with its concentration around 10 mg/g, the reproducibility is somewhat lower. Apart from variations in time in the stability of the instrument, positioning of the sample, etc., another possible but very minor source of error in this experiment could be variations in hair length due to changes in air hu- midity. The difference in length between a dry and a wet fiber is not much more than 1%. A strong rise in the concentration of K occurs some 3-4 mm from the root. This corresponds approximately to where the fiber has lost the outer root sheath, i.e., is exposed to the environment and thus effectively is brought above the skin surface. It is then exposed to the excretion of the skin glands and to the environment, and hence all data from measurements in that part of the hair are likely to suffer from contamination effects. Another aspect of reproducibility is the observation that the standard deviation for zinc in the present study is much lower than in the preceeding PIXE study based on the same material (9). Earlier studies indicate that zinc has a relatively even distribution over the cross section, while the sulfur concentration varies. This can partly explain the discrepancy in measured sulfur values between the studies, as the data in the PIXE-based study primarily arise from the hair surface. The sulfur content of the intact exocuticle has been estimated to be about 11% (25). Selecting the empirical median value from each group of hairs (each person) and exclu- sively including these data in the database make it more likely that occasional errors have been excluded. One type of problem that might be solved by this means is the alteration of analytical data by particles occurring on the hair surface. In the hair groups, i.e., the three or six hairs from each person, there is, however, a tendency of co-variation. Thus, if a particular fiber was shown to be rich in a certain element, other hairs of the same sample group were also high in this element, as can be seen when comparing mean and median data (Tables I and II). This correlation has not been studied in detail. Monovalent ions appear to be relatively loosely bound to the hair fiber material. As was mentioned above, the K concentration can be expected to vary a lot between different sites on a fiber (Figure 4). The monovalent ions K and CI are highly water-soluble and are easily washed out when cleansing the hair in distilled or preferably in deionized water. When surfactants are used, they should be of the non-ionic type for obvious reasons. To a certain extent, this is also true for Ca, but Ca is sometimes present in tap water in considerable amounts and can thus be absorbed also. CONCLUSIONS The present investigation introduces a new energy-dispersive X-ray technique for el- emental analysis of biological materials, here represented by hair fibers. The method proves to have distinct advantages over particle probes in that it causes no loss of material during the analyses, with the doses used. This results in data corresponding to "live concentrations" at a level of sensitivity that matches that of PIXE (proton-induced X-ray emission) analysis. The particle probes produce characteristic secondary X-ray emission when electrons or protons are retarded or stopped by elements in the specimen.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)