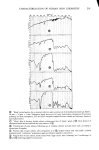
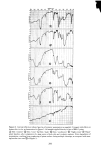
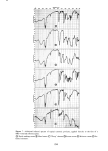
J. Soc. Cosmet. Chem., 29, 339-352 (May 1978) Interaction of keratinous substrates with sodium lauryl sulfate.. II. permeation through stratum corneum J. A. FAUCHER and E. D. GODDARD Union Carbide Corporation, Tarrytown, NY 1059I. Received September 9, 1977. Synopsis Neonatal rat STRATUM CORNEUM was used as a model membrane to investigate PERMEATION through mammalian skin. Passage of materials through these membranes was determined by use of radio- tagged compounds and by spectrophotometric analysis. The anionic surfactant SODIUM LAURYL SULFATE penetrates the stratum corneum even at low concentrations. The diffusion constant for this process is about 10 -•ø cm"/sec, compared to 10 -6 cm"/sec for free diffusion in water. This SURFACTANT is bound to the skin in large amounts, up to 50% by weight at high concentrations. Pretreatment of the membrane by a cationic cellulose polymer (which is itself strongly sorbed) greatly reduced the amount of surfactant which passed through the membrane. INTRODUCTION Considerable study has been made in the past of the effect of surfactants on the permeability of mammalian skin. For example, Bettley and Donoghue (1) showed that soap not only penetrates the skin barrier, but also makes the barrier more permeable to water and other solutes. A number of studies thereafter emphasized the increased per- meation of water or salts (2-6). Relatively little, however, has appeared on the permea- tion of surfactants themselves through skin. Perhaps the first quantitative study was by Blank and Gould in 1959 (7) showing that anionic surfactants in low concentration penetrated human epidermis with difficulty. Blank's subsequent work on a cationic surfactant indicated virtually no penetration at all (8). With somewhat greater con- centrations, Scala et al. (9) and Howes (10) demonstrated more permeation by various anionic surfactants. All of these previous studies were quite limited in terms of the range of concentrations studied and the time scale investigated yet both factors have been specifically recognized to be of great importance in the permeation process (5). The authors' interest in the permeation of surfactants through skin arose in con- nection with a clinical study of antiirritation effects of a cationic polymer (11). A detailed study of both sorption and permeation of the anionic surfactant sodium lauryl sulfate has been made with the aim of clarifying the mechanism of action of these ob- served protective effects. 339
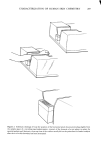
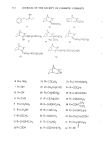
340 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS EXPERIMENTAL Stratum corneum of neonatal rats was used as a model for human skin. Live young rats one or two days old were obtained from Marland Breeding Farms, West Milford, New Jersey. The animals were sacrificed by being placed in an atmosphere of CO2 for several hours after death the whole skin was removed by a surgical scalpel. The skin was placed in a desiccator jar and exposed to ammonia vapor for 1 to 3 hr. Following this, the skins were put in water and the epidermal layer was gently separated from the dermis. The epidermis so obtained was floated on the surface of a pan of water. After an hour the membrane was removed by bringing up a metal screen under it. The membrane was placed top down on a wet paper towel and the screen removed. At this point the Malpighian layer could be gently scraped off, leaving the desired stratum cor- neum on the towel. The paper and stratum corneum were placed again in water until separation occurred. The stratum corneum layer was recovered by a small Teflon screen and air-dried. Isolation of the stratum corneum follows a method outlined for us by E. J. Singer and E. Bolsits of Lever Brothers Co., Edgewater, New Jersey. A typical piece of stratum comeurn was about 25/•m thick and 5 x 6 cm in area. It weighed about 20 rag, corresponding to a density of 0.7. The permeability cell used was modeled after a description by Loveday (12), and is shown schematically in Figure 1. The temperature of the experiments was that of the laboratory, 23øC + iøC. The surfactants used in this study were: Tergitol 15-S-9 (Union Carbide Corp.), the 9 mol ethoxylate of a secondary C H to C •a alcohol. PERMEABILITY CELL AFTER LOVEDAY (1961) SOLUTION- SKI N --STIRRER BAR Figure 1. Schematic drawing of permeability cell
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)