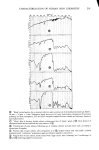
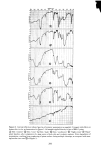
330 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS Z "' 6- o 5- z 4- 3- 2- 1- BLEACHED VIRGIN BROWN ß I 2 3 HOURS Figure 5. Sorption of 10% sodium lauryl sulfate by bleached and virgin brown hair ing activity of the monomer in this region. Unfortunately it is very difficult to make direct measurements that are unambiguously related to the SLS monomer concentra- tion in the region well above the CMC. Thus, while it is not clear that monomer concentration in fact does increase there, the sotpriori data shown here are consistent with such an interpretation and they show the same kind of phenomenon found in the dialysis experiments of Mysels. THE DIFFUSION PROCESS From the uptake vs. •/t curves a rough estimate can be made of the diffusion constant of SLS in the keratinous medium, either hair or skin. For hair, the formulation com- monly employed is that which represents diffusion into an infinitely long cylinder at short times (9): Q (t) _ 4 / Dt -- -- •(•) r w
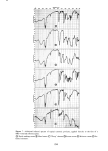
SORPTION OF KERATINOUS SUBSTRATES 331 8O •- 6O 15% 10% 5% 2O 0 0 0.1% 1 2 HOURS Figure 6. Data of Figure 4 vs. •/t where r is the radius of the hair fiber, D is the diffusion constant p4nd librium" uptake, i.e., at very long times. Ifr is taken as 25 x 10-- cm mate is made for Q (o•) by measuring uptake after several days, bleached hair D = 1 to 3 x 10-" cm2/sec and for undamaged hair cm2/sec. Within the uncertainty of estimation of values for Q (•) the diffusion constant D were found to vary only slightly for the concentratioxtheIofrangecloselya(15),wool,magnitudesGriffithforDforesti-"equi-theroughobtainsisa(o=)andoneQ between 0. 1 and 10%. They compare well with values reported by 10-" cm2/sec, and by Chen (16), 4 x 10-" cm2/sec, in both cases related substrate. These authors made use of the formula cited above. In the case of skin, we prefer to use the well known formula of A. V. Hill (see reference 7 for a derivation): where Q is the uptake in g/cm •, Co is the external concentration in g/cm s, t is the
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)