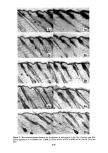
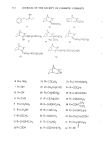
322 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS eindruck verblagt. Dies mfigte auch ffir endo-konfigurierte Substanzen am C2-Atom zutreffen. Die Clbereinstimmung der Ergebnisse mit der Amoore'schen Theorie ist bei der Verbindungsklasse der Isocamphane gegeben. Zusammenfassung An Hand von verschiedenen Isocamphanverbindungen wurde gezeigt, inwieweit die stereochemische Theorie von Arnoore zur Erklfirung des Geruchseindruckes angewendet werden kann. Mit Hilfe des Schatten- korrelationstestes wurden die Molekfile gegenfiber der Standardsubstanz 1,8-Cineol vermessen. Die l•bereinstimmung der Ergebnisse mir anderen Molekfilparametern wurde geprfift. Die kugelig geformten Molekfile dieser Reihe rufen emen camphrigen Geruchseindruck hervor und es nimmt dieser reit zunehmender Asymmetrie der Molekfile ab. (3} (4} (71 (91 I101 Illl (12 [13 (14 (15 (16 (17 (18 119 (20 (21 [22 [23 [24 (251 Literatur Amoore, J. E., Molecular Basis of Odor. Charles C. Thomas Publ. Springfield 1970. Klissenbauer, E., Diplomarbeit, Universit& Wien, 1977. Mazziotti, A., Nature 250, 645 [197211. Reckhard, H., Erd61 u. Kohle 11,234 [19581. Cavalli-Sforza, L., Biometrie, Grtmdzfige biolog. med. Statistik, G. Fischer Vlg., Stuttgart 1972. Ther, L., Gnmdlagen der experimentellen Arzneimittelforschung, Wiss. Vlg. Ges. m. b. H., Stuttgart [1965}. Nametkin, S., Ann. Chem. 438, 185 [19241. Ipatiev, W., Bet. dtsch. chem. Ges. 45, 3206 [1912[. Hana, G. W., Dissertation, Universit& Wien, 1971. Beckmann, S. und Geiger, B., Chem. Bet. 94, 1910 [1961}. Buchbauer, G., Synthesen i.d. Isocamphanreihe 5. Mitt., Mh. Chem., im Dmck. Komppa, G., Ann. Chem. 366, 75 (19091. Hana, G. W., Buchbauer, G. und Koch, H., Mh. Chem. 107, 945 11976). Lipp, P., Dessauer, H. und Wolf, E., Ann. Chem. 525, 271 [1936[. Arnoore, J. E. und Venstrom, D., J. Food Sci. 31, 118 [19661. Aschan, O., Ber. dtsch. chem. Ges. 41, 1092 [1908}. Buchbauer, G., Hana, G. W. und Koch, H., Mh. Chem. 107, 387 [1976[. Buchbauer, G., Hana, G. W. und Koch, H., Arch. Pharm., im Dmck. Langlois, G., Ann. Chim. [9], 12, 290 11919}. Lipp, P., K/tippers, P., Holl. M., Bet. dtsch. chem. Ges. 60, 1575 [1927}. Buchbauer, G., Sci. pharm., 45, 196 (1977). Buchbauer, G., Mh. Chem., im Dmck. Buchbauer, G., Dissertation, Universitat Wien, 1971. Buchbauer, G., Tetrahedron Letters 1977, 7. v. Soden, H., Arch. Pharm. 238, 353 11900} Ruzicka, G. und Thomann, G., Helv. Chim. Acta 18, 355 [1935[. Jamasaki, H., Katuhara, J. und Yamamoto, N., Koryo 1970, 33.
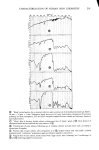
J. Soc. Cosmet. Chem., 29, 323-337 (May 1978) Interaction of keratinous substrates with sodium lauryl sulfate.. I. sorption J. A. FAUCHER and E. D. GODDARD Union Carbide Corporation, Tarrytown, NY 1059I. Received September 9, 1977. Presented at Annual Seminar Meeting, Society of Cosmetic Chemists, May I977, Montreal, Canada. Synopsis Use was made of radiotagged SODIUM LAURYL SULFATE (SLS) to determine its sorption by skin and hair. In the initial stages uptake is linear in square root of time, indicative of a diffusion process. The uptakes determined by radiotagged SLS were successfully correlated with data from a simple gravimetric method and showed that the latter procedure can be used satisfactorily under certain conditions when radiotagged com- pounds are not available. The influence of some additives on the SORPTION of SLS was studied. Salt increases the sorption, while nonionic SURFACTANTS (which are not themselves sorbed) substantially depress it. Finally, the relation of the sorbed SLS to water of hydration of KERATIN is examined. It is con- cluded that most, if not all, the sorbed material is bound to keratin, rather than existing in an "internal" solu- tion. INTRODUCTION Surl5•ctants and soaps are known to be irritating to the skin and under extreme condi- tions can have adverse effects on hair. Scientific study of the action of these materials is hampered by a lack of data on their uptake by various keratin substrates. The avail- ability of sodium lauryl sulfate (SLS) in radiotagged form makes it relatively easy to study the kinetics of sorption of this model surfactant over a wide range of concentra- tions and times, and to explore the various effects of additives. There are few data in the literature concerning the sorption of surfactants by human hairß Two studies (1,2) have dealt with long-chain quaternary ammonium halides (typical cationic surfactants), but both were conducted at concentrations well under 1% A brief ra•di•otracer stud was made of sodium ac¾1 sarcosinates (3) in which the ß _ .... r_a_cer•_ st• concentration was as h•. However, n•ot•h_ing ba•s_a_p_.peared dealing with non- ionics, amphoterics or more common anionics such as sodium lauryl sulfate and its ethoxylates nor have concentrations in the range of 10% been investigated, cor- responding to the actual strength of shampoos. The situation is similar for the substrate skin. There is a large, medically oriented litera- ture on the percutaneous absorption of surfactants-particularly anionics like SLS, but the uptake of surfactant by the skin is not usually considered in these works. Two not- 323
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)