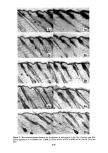
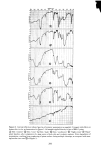
PERMEATION OF KERATINOUS SUBSTRATES 349 reducing skin irritation caused by sodium lauryl sulfate. It seems likely that sorption or permeation (or both) of the surfactant must be influenced by the presence of the polymer. It is true that the polymer does interact with SLS in solution (20). However the amount placed on the skin in the clinical studies is too little to explain the observed phenomena either by interaction or by film formation. Experiments on the effect of Polymer JR upon sorption of SLS were carried out in tWO ways: (1) with the polymer present in the SLS solution and (2) by application to the stratum corneum, i.e., soaking for 1 hr in a 1% solution. Neither had any effect on the sorption of SLS. Thus Figure 9 shows data for 0.1% SLS with pretreatment. Other concentrations of SLS gave the same results. However, permeation experiments did show a pronounced effect, one that was concentration-dependent. Figure 10 shows data for preapplication of Polymer JR (1• hr 0.1% SODIUM LAURYL SULFATE 30 o z o I I o I 2 HOURS Figure 9. Effect of Polymer JR on sorption of sodium lauryl sulfate. Solid line: untreated. Dashed line: pretreatment for 1 hr with 1% Polymer JR
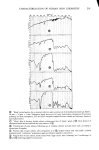
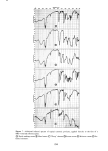
350 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS 160 140 120 E 100 80 60 - ß 20 ,,//• ' ..o- 0 20 40 60 80 100 120 HOURS Figure 10. Effect of pretreatment by Polymer JR on permeability of 0.1% sodium lauryl sulfate. Solid line: untreated. Dashed line: pretreated for 1 hr with 1% Polymer JR of 1% solution) and permeation of 0.1% SLS a time of some 60 to 70 hr is necessary before the permeability of the untreated membrane begins to rise sharply above that of the treated skin. This effect is reproducible as the repeated curves show. Even more striking are the results of Figure 11 at 10% SLS. Here significant differences are al- ready evident in less than 10 hr. An exact correspondence cannot readily be made to the conditions and times of the clinical testing cited above (11) but it is clear that these latter experiments involving prolonged occlusion and lasting for several days must represent many hours of effective contact with concentrated surfactant. These experiments of sorption and permeation begin to suggest a plausible mechanism for the action of the polymer on the stratum corneum. The polymer does not act pri- marily as a barrier to penetration of the surfactant since it has no influence on sorption. However, it does help to maintain the physical integrity of the membrane. That is, it seems to slow down the changes in structure which cause the greatly increased permea- tion as time goes on (Figures 7 and 8). The exact means by which the polymer strengthens the stratum corneum is not known at this time but it has been established
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)