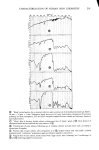
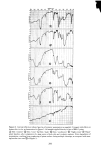
336 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS 60 30 , 2o 10 0 I 2 3 4 5 6 7 8 9 % SLS 10 Figure 11. One hour uptakes of sodium lauryl sulfate in stratum corneum: solid line, as measured experi- mentally dotted line, calculated by assuming that water of hydration has the same composition as the external solution uptakes of SLS at ! hr with the calculated uptakes at each concentration assuming that the water of hydration or "internal" solution has the same concentration as the "external" •olution. The latter curve was calculated from swelling data obtained at a 1 hr exposure for a number of concentrations of SLS. It will be noted that the uptakes at low concentrations (below the CMC) are much greater than the calculated uptakes. But at high concentrations (above 5 %) the calculated uptakes are larger than the measured ones. This lack of agreement clearly shows that the internal solution does not have the same concentration as the external one. It does not exclude the possibility, however, of some SLS monomer existing in free solution inside the stratum corneum. The evidence above suggests that this possible state is unlikely to amount to more than a small frac- tion of the measured uptake. In this connection it may be recalled that collagen and protein in general can bind large amounts of SLS. Nelson (22) has shown that as much as 1.1 to 2.2 g of SLS/g of protein can be bound under the most favorable conditions. Thus the inference that all of the SLS uptake reported here is bound to the keratin is not unreasonable. More light could be shed on this point by a detailed NMR study of the state of the lauryl sulfate anion in hydrated stratum corneum and hair. CONCLUSIONS It has been shown that the uptake of anionic surfactants by hair and stratum corneum membranes is appreciable. With sodium lauryl sulfate, SLS, the uptake increases markedly with concentration even above the critical micelie concentration, and it also
SORPTION OF KERATINOUS SUBSTRATES 337 increases in the presence of added salt but decreases in the presence of added nonionic surfactant. Lauryl ether sulfates are sorbed to a lesser extent than SLS and their uptake decreases with ethylene oxide content. By comparison of sorption data obtained by radiometric and gravimetric techniques, it has been demonstrated that a simple weighing technique can be employed for measur- ing the uptake of surfactants and simple salts, in view of their relatively high sorption values. REFERENCES (5) (6) (7) (8) (9) (10) (11) (12) (13) (14) 15) 16) (17) (18) (19) (1) G. V. Scott, C. R. Robbins and J. D. Barnhurst, Sorption of quaternary ammonium surfactants by hair, J. Soc. Cosmet. Chem., 20, 135 (1969). (2) P. Finkelstein and K. Laden, The mechanism of conditioning of hair with alkyl quaternary ammonium compounds,Appl. PolymerSymp., No. 18, 673 (1971). (3) M. F. Nelson, Jr. and D. Stewart Jr., The adsorption of N-acyl sarcosines on various protein materials, J. Soc. Cosmet. Chem., 7,122 (1956). -• (4) S. P. Harrold and B. A. Pethica, Thermodynamics of the adsorption of small molecules by proteins, Trans. Faraday Soc., 54, 1876 (1958). I. H. Blank and E. Gould, Penetration of anionic surfactants into skin, J. Invest. Dermatol., 33,327 (1959). H. E. Garrett, Detergents--what they are and how they work, Trans. St. John Hosp. Dermat. Soc., 51, i66 (i965). J. A. Faucher and E. D. Goddard, Sorption of a cationic polymer by stratum corneum, J. Soc. Cosmet. Chem., 27,543 (1976). E.J. Singer, P. C. Wegmann, M.D. Lehman, M. S. Christenson and L.J. Vinson, Barrier development, ultrastructure, and sulfhydryl content of the fetal epidermis, J. Soc. Cosmet. Chem., 22, 119 (1971). P. B. Sram and H. J. White,Jr., The application of radiochemical techniques to the study of the interac- tion of hair fibers with aqueous solutions, Text. Res. J, 24, 785 (1954). J. A. Medley and M. W. Andrews, The effect of surface barrier on uptake rates of dye into wool fibers, Textile Res. J, 29,398 (1959). J. A. Medley and M. W. Andrews, The kinetics of wool dyeing, Text Res. J., 30, 855 (1960). N.H. Leon, Structural aspects ofkeratin fibers, J. Soc. Cosmet. Chem., 23,427 (1972). W. C. Preston, Some correlating principles of detergent action, J. Phys. Chem., 52, 84 (1948). M. Abu-Hamdiyyah and K. J. Mysels, The dialysis of sodium dodecyl sulfate, J. Phys. Chem., 71,418 (1967). J. Griffith, "The Uptake of Sodium Dodecyl Sulfate by Wool," Proc. 3rd Internat. Congr. Surface Activ., Cologne, 1960, Vol. 4, pp 28-36. J. C. Chen, The Interactions of an Anionic Surfactant with Keratin Fibers, Ph.D. Thesis, Princeton University, Princeton, New Jersey, 1971. I. J. Lin, "Hydrophobic Properties of Long-Chain Ionic Surfactants," in Colloid and Interface Science, Vol II, M. Kerker, Ed., Academic Press Inc., New York, New York, 1976, pp 431-443. M. J. Schick and D. J. Manning, Micelie formation in mixtures of nonionic and anionic detergents, J. Am. Oil Chem. Soc., 43, 133 (1966). A. W. Finkstein, "Eye Irritation Studies on Some Common Shampoo Surfactants," paper presented at the Annual Scientific Meeting of the Society of Cosmetic Chemists, New York, New York, December 1976. (20) J. Clifford and B. Sheard, Nuclear magnetic resonance investigation of the state of water in human hair, Biopolymers, 4, 1057 (1966). (21) R. L. Anderson, J. M. Cassidy, J. R. Hansen and W. Yellin, Hydration of stratum corneum, Biopolymers, 12, 2789 (1973). (22) C. A. Nelson, The binding of detergents to proteins, J. Biol. Chem., 246, 3895 (1971).
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)