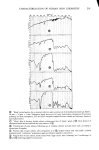
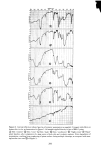
332 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS 60- ' • • • •lm, • •'• • • •'-" • BLEACHED HAIR O• I 2 3 4 5 6 7 8 9 10 CONCENTRATION OF SLS IN PERCENT Fiõure 7. Concentration dependence ooe sorption rate: uptake ooe sodium ]auryl suloeate by stratum comeurn and bleached hair _z 40 •- 30 20 10 time in seconds and D is the diffusion constant. The diffusing species is assumed to be the SLS monomer hence Co corresponds to the total solution concentration only in the range below the CMC (0.24%). Using data at 0.1 and 0.2% one obtains by this formula D = 3 to 7 x 10 -9 cm2/sec, about three orders of magnitude higher than in und&maged hair but still considerably lower than for SLS in water. SORPTION OF SODIUM LAURYL ETHER SULFATES Having measured the sorption of 10% SLS by a simple weighing procedure, it was of interest for comparison to determine the uptakes of closely related surfactants: Standapol ES-2, ES-3 and 130-E. These are, respectively, the 2, 3 and 12 tool ethoxy- lates of SLS and they represent a chemical series which increases in ethylene oxide content. Radiotagged samples of these materials are not available, so the gravimetric procedure described above was used. In Figure 8 their uptakes vs. time are plotted and compared to SLS. Bleached hair was the substrate. There is clearly a reduction in sorp- tion with increasing number of ether groups in the surfactant molecule--a reduction which also persists on a molar plot. There are several possible explanations for this ef- fect. The simplest is that the molecules increase in size in this series and hence have more difficulty getting into the hair structure. Also very convincing is the fact that the CMC decreases markedly for these compounds as the ethylene oxide content increases (17). Thus the monomer concentration available for diffusion will be a decreasing func-
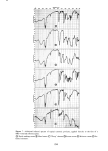
SORPTION OF KERATINOUS SUBSTRATES 333 Z SLS ES-2 ES-3 1 i• 130 E I I O0 2 4 6 8 10 12 HOURS Figure 8. Sorption of sodium lauryl ether sulfates by bleached hair from 10% solution tion also. This decreased sorption correlates well with the well known milder prop- erties of highly ethoxylated anionic surfactants compared to SLS. THE EFFECT OF ADDITIVES A feature of SLS sorption is that it is strongly influenced by the addition of certain other compounds. For example, sodium chloride generally causes an increase in sorp- tion. This effect is well known from work on wool (15,16). It is even more pronounced with stratum corneum as the substrate, as the data in Figure 9 show. Harrold and Pethica (4) found the same phenomenon with finely divided epidermal keratin. Salt decreases the CMC of SLS, so the monomer concentration will be lowered in its presence. It seems, therefore, that the salt must act on the substrate in a way that makes it more available to the surfactant or by a nonspecific electrical screening effect. On the other hand, the addition of a nonionic surfactant such as Tergitol 15-S-9 considerably decreases the sorption of SLS, both for hair and skin. This is not due to competition between the two surfactants for sites in the keratin, because the nonionic material is hardly sorbed at all by itself. Instead it is known that mixed micelies of the two surfactants are formed. For a very similar system Schick and Manning (18) have
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)