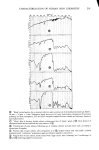
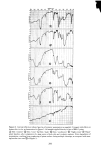
334 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS 1% SLS + 1% NaCl 1% SLS 40 • 30- 20 lO I øo 1 2 3 4 5 HOURS Figure 9. Effect of salt on the sorption of sodium lauryl sulfate shown that even small additions of a nonionic surfactant have a large effect in lowering the CMC of sodium lauryl sulfate. This brings about a lowering of the SLS monomer concentration and, hence, lower sorption. Figure 10 demonstrates the effect in a strik- ing way. This furnishes a physico-chemical explanation of the findings of Finkstein (19) who showed that a reduction of irritation of anionic shampoos occurs on the addition of nonionic surfactants in spite of the fact that the total surfactant concentration increased. In this case, lower irritation is attributed to decreased sorption of the anionic surfactant by proteins of the skin and cornea. RELATION OF SORBED SURFACTANT TO WATER OF HYDRATION Both hair and stratum corneum absorb water when placed in solution. It is therefore conceivable that some, if not all, of the sotbed surfactant may be present as a solute in this "internal" solution, rather than being truly bound to the keratin. The analytical method employed here does not distinguish these cases. It is not easy to decide this point conclusively, but the available evidence indicates that the surfactant is bound to the substrate.
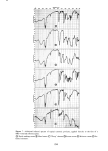
SORPTION OF KERATINOUS SUBSTRATES 335 NO TERGITOL WITH 1% TERGITOL : j ,,/ WITH 5% TERGITOL I I O0 I 2 3 4 5 6 HOURS Figure 10. Effect of a nonionic surfactant on the sorption of sodium lauryl sulfate by bleached hair For undamaged hair the water of hydration amounts to about 35 % by weight of the dry substrate this water is absorbed in less than 1 min. However, the SLS sorption, as shown in Figure 1, goes on for many hours. Furthermore, at low solution concentration of SLS the ultimate amount of surfactant taken up by hair can amount to ten times as much as would be calculated solely from the "external" solution. A study has been made by NMR of the mobility of water in hydrated hair (20). In this work it was found that such water is quite immobile and tightly bound to the keratin. It seems unlikely that SLS can exist as a normal solute in such an environment. The case of stratum corneum is somewhat different in that this substrate absorbs as much as 1000% of its own dry weight over a period of many hours when immersed in aqueous solution. A detailed study of this water (21) shows, however, that much of it is quite restricted in mobility and probably located in the interior of the keratin cells. Again, it seems unlikely that this water ofhydration can behave like the bulk "external" solution in particular, the existence of ordinary micelles therein is improbable because of exclusion effects. Figure 11 shows two curves which compare the actual measured
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)