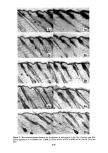
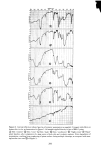
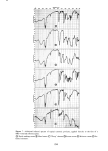
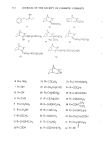
282 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS that HDO was localized in the skin and resistant to metabolization while IPM was easily distributed into the organs and metabolized. When cosmetic bases are considered relative to their safety, their reactivity with or their localizing nature in the skin should be studied simultaneously with their macro- scopic skin-irritation properties. REFERENCES (1) (2) (3) (4) (5) (6) (7) (8) (9) (10) (11) (12) (13) (14) (15) (16) (17) (18) (19) (20) (21) (22) (23) T. Rutherford and J. G. Black, The use of autoradiography to study the localization of germicides in skin, Brit. J. Dermatol., 81 (Suppl. 4), 75 (1969). J. G. Black and D. Howes, Percutaneous absorption of Triclosan from toilet preparations, J. Soc. Cosmet. Chem., 26, 205 (1975). B. Calesnick, C. H. Costello, J.P. Ryan and G. J. DiGregorio, Percutaneous absorption of hexachlorophene following daily whole body washings, Toxicol. Appl. Pha rmacol., 32, 204 ( 1975). J. R. Taylor and K. M. Halprin, Percutaneous absorption of salicylic acid, Arch. Dermatol., 111,740 (1975). J. W. Goldzieher and R. E. Baker, The percutaneous absorption of estradiol-1713 and progesterone, J. Invest. Dermatol., 35,215 (1960). R. J. Feldmann and H. I. Maibach, Percutaneous penetration of steroids in man, J. Invest. Dermatol., 52, 89 (1969). R. C. Wester and H. I. Maibach, Percutaneous absorption in the rhesus monkey compared to man, Toxicol. Appl. Pharmacol., 32,394 (1975). W. Montagna, Penetration and local effect of vitamin A on the skin of the guinea pig, Proc. Soc. Exp. Biol. Med., 86, 668 (1954). A. E. Schaefer, H. L. Sassaman, A. Slocum and R. D. Greene, Absorption of topically applied vitamins, J. Nutr., 59, 171 (1956). I. H. Blank, E. Gould and A. Theobald, Penetration of cationic surfactants into skin, J. Invest. Dermatol., 42,363 (1964). J. Scala, D. E. McOsker and H. H. Relier, The percutaneous absorption of ionic surfactants, J. Invest. Dermatol., 50, 371 (1968). D. Howes, The percutaneous absorption of some anionic surfactants,J. Soc. Cosmet. Chem., 26, 47 (1975). M. Ainsworth, Methods for measuring percutaneous absorption, J. Soc. Cosmet. Chem., 11, 69 (1960). M. Barr, Percutaneous absorption,J. Pharm. Sci., 51,395 (1962). R. T. Tregear, "Physical Functions of Skin," Academic Press, New York, 1966, pp 1-52. P. Grasso and A. B. G. Lansdown, Methods of measuring, and factors affecting, percutaneous absorp- tion, J. Soc. Cosmet. Chem., 23,481 (1972). B. Id son, Percutaneous absorption,J. Pharm. Sci., 64, 901 ( 1975). S. Ullberg, Autoradiographic localization in the tissues of drugs and metabolites, Biochem. Pharmacol., 9, 29 (1962). C. P. Petroff, H. H. Patt and P. P. Nair, A rapid method for dissolving tissue for liquid scintillation counting, Int. J. Appl. Radiat. Isotop., 16, 599 (1965). M. Suzuki, H. Komatsu, K. Asaba and S. Kato, Application of whole body autoradiography to measur- ing percutaneous absorption, J. JCCA, 9, 16 (1974). M. J. Bartek, J. A. LaBudde and H. I. Maibach, Skin permeability in vivo: comparison in rat, rabbit, pig and man, J. Invest. Dermatol., 58, 114 (1972). Unpublished data. Unpublished data.
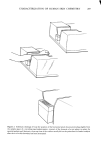
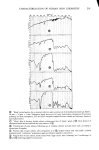
j. Soc. Cosmet. Chem., 29,283-306 (May 1978) Noninvasive, rapid characterization of human skin chemistry in situ R. E. BAIER Calspan Corporation, Buffalo, NY 14221. Received May 26, 1977. Presented at Annual Meeting, Society of Cosmetic Chemists, December 1976, New York, New York. Synopsis The experimental yield of internal reflection spectroscopic and contact angle techniques applied to LIVING HUMAN SKIN is demonstrated for natural, cosmetically treated and wounded epidermis. The critical SUR- FACE tension and chemical composition of clean human skin surfaces is provided, along with spectral data bearing on the efficacy and quality of cosmetics, the depth profile of skin moisture, and the CHEMICAL NATURE ofexudates from epidermal wounds. INTRODUCTION Professional groups, individual research chemists, consumer safety advocates and legis- lative bodies are increasingly calling attention to the potential hazards, and unverified claims for quality and efficacy, of cosmetic and therapeutic products applied to skin surfaces. Despite the pressures generated, even modern industrial research labora- tories assessing the influence of various reagents on human skin continue to assume the end effects of frequent skin contact with certain chemical types without proof of the validity of such assumptions. For example, surfactant-containing liquids are assumed to damage human skin by excessive removal of skin lipids, deposition of the surface-active agents themselves (upon or immediately subjacent to the skin surface) and denatura- tion of the proteinaceous structures in the epidermal layers. These actions are sup- posed to lead to the abnormal features of human skin encompassed by clinical symptoms of roughness, scaliness, unsightly wrinkling, irritation and dermatitis. Since many of the functional and aesthetic qualities of human skin are attributed to the skin moisture balance, a continuing goal of most cosmetic "moisturizing" preparations is to limit or prevent transepidermal, moisture losses. Here, also, the in situ functions of the main ingredients of such preparations have not been convincingly demonstrated--merely assumed--to play a role in moisture retention, rather than simply lubricating and/or plasticizing the skin through ester imbibation. It is no longer necessary to accept without proof such assumptions in the development and certifica- tion of benefits for cosmetic or skin-healing products. Direct, noninvasive, relatively 283
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)