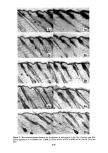
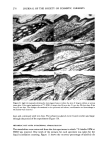
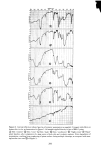
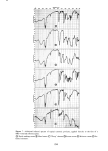
PERMEATION OF KERATINOUS SUBSTRATES 341 Barquat MS-100 (Lonza, Inc.), myristyldimethylbenzyl ammonium chloride. Sodium lauryl sulfate (SLS) was obtained as a pure white crystalline powder from BDH Chemicals. Analyses of the first two materials were made spectrophotometrically, by absorption at 337 nm for Tergitol and at 262 nm for Barquat. In these cases, the small amount of protein leached from the stratum comeurn does not interfere with absorption peaks of the surfactants. Radiotagged material was purchased from Amersham-Searle (Des Plaines, IL) in the form of small individual ampoules. Each ampoule contained 2.47 mg of SLS with an activity of 100 microcuries. The tag was present as the S-35 isotope and is thus in the anionic part of the surfactant. Solutions of desired concentration were made up of the nonradioactive powder and one ampoule was added with stirring. For permeability ex- periments a small sample (0.1 g) of the water in the lower part of the cell was removed by pipette and put in a 1-oz counting vial filled with the scintillant liquid, Instagel. Ra- dioactivity was determined by scintillation counting in a Packard 3255 Counter. For sorption experiments skin samples of about 2 mg each were placed in 20 ml of tagged solution for various set times, removed and rinsed twice for a few seconds with distilled water to remove entrained solution. The skin was then dissolved in UNISOL and a scintillant cocktail added. Radioactivity was again determined in the Packard Counter. Polymer JR is a quaternary nitrogen-containing cellulose ether (13). The JR-400 grade was used for the experiments reported here. Its approximate molecular weight is 400,000. NOTE ON CALCULATIONS For •biological membranes such as skin, whose thickness is difficult to measure, it is convenient to work with the apparent permeability defined by (14) Q/A = P Ac t, [1] where Q is the amount of solute which penetrates through area A in time t P is the ap- parent permeability constant and Ac is the difference in concentration between the two sides of the membrane. For this work Ac is taken to be simply the initial concentration of the upper solution, since the concentration in the stirred water is always close to zero. An idealized Q vs. time curve is shown in Figure 2. There is an initial slow growth of Q leading to a straight line portion, the extrapolation of which back to the time axis gives the "lag" time, To. From this time a diffusion constant, D, can be calculated by (14) D = h2/6To [2] where h is the thickness of the swollen membrane. The plot illustrates how both P and D can be derived from permeability data. For purposes of calculation h has been assumed here to be 50/•, which is about twice the dry thickness of the stratum cor- neum. (It should be appreciated that individual membranes vary in thickness and that the swollen thickness changes slowly with time.) In the ideal case, the apparent permeability is related to the diffusion constant, D, by the equation:
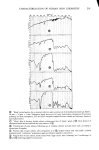
342 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS Z SLOPE • - P, cm/sec D(cm2/sec) = h 2 6T o T 0 TIME Figure 2. Idealized permeation curve P = (KD)/h [3] where K is a unitless partition coefficient, being the ratio at equilibrium of the concentration of solute in the membrane to the concentration of solute in solution (15). This allows determination of K, or of P by two different methods ifK is known. RESULTS AND DISCUSSION In view of the fact that each permeation run uses a different individual skin, some general comments on reproducibility are appropriate. Generally speaking, precision was found to be good. For example, Figure 3 shows three separate stratum corneum samples treated for permeability to 10% SLS, a very aggressive environment. Better results could be obtained at lower concentration: Figure 4 contains data for two dif- ferent runs at 0.5% SLS. However, it was not always possible to obtain such good agreement, particularly at the higher concentrations where the membranes are substantially dissolved with time. Occasional skins showed immediate passage of
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)