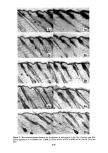
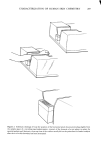
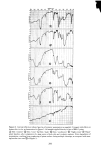
288 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS CONTACT POTENTIAL FROM VIBRATING REED ELECTRODE STUDIES INFRARED RADIATION POLARIZED L,G.T l•'-' •-, OPTICAL THICKNESS ,• • ',•' • FROELLTENSION ,-•'• GERMANIUM PRISM CRITICAL SURFACE FROM CONTACT ANGLE MEASUREMENTS Figure 3. Schematic views of (top) the simultaneous application of four nondestructive analytical tech- niques which sensitively characterize skin residues or cosmetic films on germanium prisms and (bottom) varying contact angle profiles which can be observed on living skin, on skin deposits, or with varying cos- metic preparations to measure important features of wetting and spreading phenomena schematically, nondestructive analytical techniques which can be utilized sequentially upon such a residue to characterize it by compositional criteria, organizational criteria (thickness and refractive index) and electrical character, in addition to the determina- tion of the surface chemical composition and surface energetics by the simple measure- ment of contact angle profiles (of sessile liquid droplets on the residual films or on the skin surfaces themselves). Numerous applications of these simultaneous, nondestruc- tive methods to biologically relevant surfaces have already been provided in a recent volume of the Advances in Chemistry series (13), including specific examples of
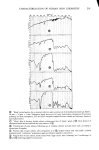
CHARACTERIZATION OF HUMAN SKIN CHEMISTRY 289 natural, cosmetically modified, old and freshly generated human skin in situ. Referring again to Figure 3, it should be noted that the internal reflection prism shown there is labelled as being constructed ofgermanium, an important requirement not only for the application of ellipsometry and contact potential measurements to the films in question but also assuring biological safety for the volunteer subjects especially when deliberate skin wounding was employed. Thallium bromide salts, such as employed in the more commonly used KRS-5 internal reflection prisms, can cause contact dermatitis and potential toxicity in such cases, while the biological acceptability of germanium seems much better (14). RESULTS WETTING AND SPREADINO ON CLEAN HUMAN SKIN Table 1 lists the variety of pure diagnostic liquids utilized in determination of the potential wettability and spreadability of cosmetic ingredients on clean human skin. These same liquids are useful in assessing the new surface condition of skin after treat- ment with specific ingredients in proofs of efficacy for cosmetic or medicinal prepara- tions. In Figure 4, the average contact angle values obtained for these liquids on the surface of the skin of a male volunteer, after that skin had been cleansed with a liquid hand soap, well rinsed, towel-dried and equilibrated in clean room conditions, are plotted in the standard Zisman format (8, 9) to yield a critical surface-tension intercept near 38 dynes/cm. This is a typical value for uncontaminated, fibrous protein prepara- tions and compares well with values already published for human skin, keratin, collagen and gelatin (15, 16). It might therefore be taken as a general case, for human skin treated by simple cleaning procedures as described here, that cosmetic or me- dicinal preparations having operational liquid/vapor surface tensions lower than 35 dynes/cm will wet and spread well upon those skin surfaces. Fhfids with lower surface tensions will give excellent coverage and appearance, while cosmetic preparations with operational surface tensions greater than 35 to 38 dynes/cm will tend to bead up, retract, or leave interstitial voids on the same skin surfaces. Table I Wettability of Clean Human Skin, In Situ Wetting Liquid and Surface Average Tension (•/LV) Contact Angle (dynes/cm, 20øC) (0 in degrees) Water 72.8 65 Glycerol 63.4 66 Formamide 58.2 51 Thiodiglycol 54.0 54 Methylene Iodide 50.8 53 Sym-Tetrabromoethane 47.5 34 !- Bro toonaphthalene 44.6 25 O-DiBromobenzene 42.0 18 1-Methylnaphthalene 38.7 24 Dic yclohexyl 33.0 0 n- Hexadecane 27.7 0
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)