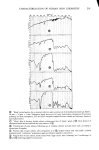
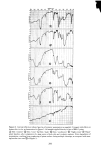
326 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS 1.6 1.4 .4 .2 o ß RADIOTRACER DATA, Na 22 BY STAM & WHITE (1954) /x GRAVlMETRIC, (1976) ß1 I I I I I 0 1 2 3 CONCENTRATION (M) Figure 1. Sorption of sodium bromide by blonde hair followed, which consists in simply patting dry the hair fibers with tissue. This effec- tively removes entrapped liquid. The weight of a tress treated in this way is surprisingly reproducible and corresponds to a kind of "fully hydrated" state. The anionic surfactant sodium lauryl sulfate (also treated below) corresponds to an intermediate case. The surfactant is fairly tightly bound by the hair substrate and is located internally as well as on the surface. However, upon very thorough washing (15 to 30 min) a third or more of it will be desorbed. Hence a compromise protocol is advisable, such as two or three successive short rinses in distilled water to remove adhering liquid and foam. With suitable care the gravimetric procedure outlined above has yielded results which are quite close to those obtained by radiotracers, as shown in the following cases: Sodium bromide, NaBr. Stam and White (9) have reported on the uptake of NaBr by undamaged blonde hair from aqueous solution, using a Na '•2 tag. Their results for several concentrations are given in Figure 1 and show a linear relation between sorp- tion and concentration. Our gravimetric data (also with undamaged blonde hair) done in duplicate at two concentrations are plotted in the same figure. The agreement is surprisingly good, considering that the hair samples are completely different. Note that
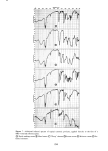
SORPTION OF KERATINOUS SUBSTRATES 327 this is a relatively favorable case owing to the large weight of sodium bromide 1 milli- mole/g corresponds to about 10% by weight. Sodium/aury/su/fate (SLS). In this instance, the uptake of SLS from 10% solution was measured on a single series (three samples for each time period). The solution was tagged with the compound described in the experimental section. First, weighing was done according to the gravimetric method above, yielding the open triangles of Figure 2. The samples were then dissolved and counted for radioactivity content following the procedure of the experimental section. This gave the solid circles of Figure 2. The agreement of the two methods is excellent indeed. Thus, for relatively favorable cases (bleached hair as substrate and large uptakes), sorp- tion of some materials by hair can be determined gravimetrically with reasonable ac- curacy. (The authors have not found it practical to adapt a similar procedure to stratum corneum as substrate.) Z 5 4 3 /' ß RADIOTRACER, SaS A GRAVIMETRIC O0 I • I • I I 2 4 6 8 10 12 HOURS Figure 2. Sorption of 10% sodium lauryl sulfate by bleached hair gravimetric and radiotracer determina- tions were made on the same samples of hair
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)