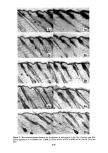
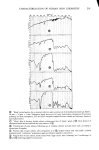
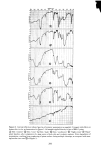
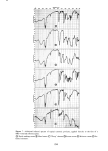
PERMEATION OF KERATINOUS SUBSTRATES 351 1.6 1.4 1.2 1.0 0.6 0.4 0.2 i 0 8 16 24 HOURS Figure 11. Effect of pretreatment by Polymer JR on permeability of 10% sodium lauryl sulfate. Solid lines: untreated. Dashed lines: pretreated for 1 hr with 1% Polymer JR that Polymer JR is itself highly substantive to stratum corneum (17). Its action may therefore be analogous to the glue-like property of cationic starches which are used to hold cellulose fibrils together in the paper-making process (21). CONCLUSION Diffusion studies have shown that penetration of (radiotagged) sodium lauryl sulfate, SLS, through neonatal rat stratum corneum is relatively rapid and increases with concentration even above the critical micelie concentration. Penetration by a cationic surfactant and a nonionic surfactant was also found but required a period of several days rather than hours as was observed for SLS. It is postulated that the high sorption and diffusion obtained with SLS are due in part to structural changes in the membrane brought about by this surfactant. These changes are mitigated by the preap- plication of a cationic polymer which was observed to reduce markedly the diffusion of SDS through the membrane.
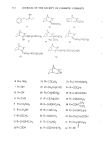
352 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS REFERENCES (1) (2) (3) (4) (5) (6) (7) (8) (9) (10) (12) (13) (14) (15) (16) (17) (18) (19) (20) (21) F. R. Bettley and E. Donoghue, Effect of soap on the diffusion of water through isolated human epi- dermis, Nature, 185, 17 (1960). F. R. Bettley, The influence of soap on the permeability of the epidermis, Brit. J. Dermatol., 73, 448 (1961). F. R. Bettley, Irritant effect of soap in relation to epidermal permeability, Brit. J. DermatoL, 75, 113 (1963). F. R. Bettley, Influence of detergents and surfactants on epidermal permeability, Brit. J. Dermatol, 77, 98 (1965). R. Scheuplein and L. Ross, Effects of surfactants and solvents on the permeability of epidermis, J. Soc. Cosmet. Chem., 21,853 (1970). G. Kiss and I. Horvath, Die wirkung des natriumlaurylsulfats auf die penetration yon electrolytes in die haut, J. Soc. Cosmet. Chem., 23,803 (1972). I. H. Blank and E. Gould, Penetration of anionic surfactants into skin, J. Invest. Dermatol, 33, 327 (1959). I. H. Blank, E. Gould and A. Theobald, Penetration of cationic surfactants intoi•skin, J. Invest. DermatoL, 42,363 (1964). J. Scala, D. E. McOsker and H. H. Reller, The percutaneous absorption of ionic surfactants, J. Invest. Dermatol., 50, 371 (1968). D. Howes, Percutaneous absorption of some anionic surfactants, J. Soc. Cosmet. Chem., 26, 47 (1975). J. A. Faucher, E. D. Goddard, R. B. Hannah and A.M. Kligman, Protection of the skin by a cationic cellulose polymer, Cosmetics and Toiletries, 92 (6), 39 ( 1977). D. E. Loveday, An in-vitro method for studying percutaneous absorption, J. Soc, Cosmet. Chem., 12,224 (1961). F. W. Stone andJ. M. Rutherford, U.S. Patent No. 3472840 (1969). For a derivation from Fick's first law see A. J. Aguiar and M. A. Weiner, Percutaneous absorption studies of chloramphenicol solutions, J. Pharm. Sci., 58, 210 (1969). R.J. Scheuplein, Mechanism of percutaneous absorption, J. Invest. Dermatol., 45,334 (1965). E. J. Singer, P. C. Wegmann, M. D. Lehman, M. S. Christensen and L.J. ¾inson, Barrier development, ultrastructure and sulfhydryl content of the fetal epidermis, J. Soc. Cosmet. Chem., 22, 119 (1971). J. A. Faucher and E. D. Goddard, Sorption of a cationic polymer by stratum corneum, J. Soc. Cosmet. Chem., 27, 543 (1976). R. J. Scheuplein and I. H. Blank, Permeability of the skin, Physiol. Rev., 51,702 (1971). M. Abu-Hamdiyyah and K. J. Mysels, The dialysis of sodium dodecyl sulfate, J. Phys. Chem., 71,418 (1967). E. D. Goddard, T. S. Phillips and R. B. Harman, Water soluble polymer-surfactant interaction, J. Soc. Cosmet. Chem., 26, 461 (1975). E. F. Paschall, "Production and Uses of Cationic Starches" in Starch, Chemistry and Technology, Vol. II, R. L. Whistler and E. F. Paschall, Eds., Academic Press, Inc., New York, New York, 1967, Chapter 16.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)