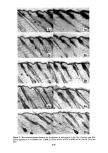
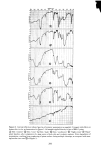
SORPTION OF KERATINOUS SUBSTRATES 327 this is a relatively favorable case owing to the large weight of sodium bromide 1 milli- mole/g corresponds to about 10% by weight. Sodium/aury/su/fate (SLS). In this instance, the uptake of SLS from 10% solution was measured on a single series (three samples for each time period). The solution was tagged with the compound described in the experimental section. First, weighing was done according to the gravimetric method above, yielding the open triangles of Figure 2. The samples were then dissolved and counted for radioactivity content following the procedure of the experimental section. This gave the solid circles of Figure 2. The agreement of the two methods is excellent indeed. Thus, for relatively favorable cases (bleached hair as substrate and large uptakes), sorp- tion of some materials by hair can be determined gravimetrically with reasonable ac- curacy. (The authors have not found it practical to adapt a similar procedure to stratum corneum as substrate.) Z 5 4 3 /' ß RADIOTRACER, SaS A GRAVIMETRIC O0 I • I • I I 2 4 6 8 10 12 HOURS Figure 2. Sorption of 10% sodium lauryl sulfate by bleached hair gravimetric and radiotracer determina- tions were made on the same samples of hair
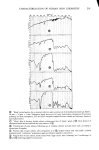
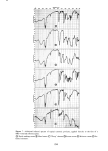
328 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS • 6- 5- 4- 3- lO% 1% 0.1% 0 I I I I 0 1 2 3 4 5 6 7 8 HOURS Figure 3. Sorption of sodium lauryl sulfate by bleached hair RESULTS AND DISCUSSION SODIUM LAURYL SULFATE Typical sorption curves at various concentrations are shown in Figure 3 for bleached hair and in Figure 4 for stratum corneum. Similar curves were obtained for undamaged hair and the uptakes in that case were approximately an order of magnitude less than found for bleached hair. For all these substrates the course of sorption follows closely a linear dependence on the square root of time, consistent with a diffusion process. In Figure 5 the data are plotted in this manner for both bleached and undamaged hair. A linear dependence is observed except for the first 15 to 30 min, where a sort of "lag time" is observed. Analogous behavior has been noted before in the dyeing of wool, a physically similar type of process (10,11). The initial lag is characteristic of the presence of a surface barrier, which in this case is postulated to be the so-called epicuticle (12). The data for stratum corneum (Figure 6) also shows good linearity in •/t but there is no evidence of a surface barrier. The slopes of the uptake - •/t lines can be regarded as a measure of the rate of sorp- tion and it is evident that these rates continually increase with concentration. Thus Figure 7 shows the uptakes at 1 hr (which are proportional to the slopes) plotted against the total surfactant concentration for stratum corneum and bleached hair. An interesting feature occurs in both cases: the rate function closely approximates two straight lines which intersect at the critical micelie concentration, CMC, i.e., the point
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)