SORPTION OF KERATINOUS SUBSTRATES 325 has been worked out for materials not available in tracer form. In studies of hair care products such as proteins, polymers and conditioners it is often of interest to de- termine the amount of material which is sorbed by the hair. The high cost of human hair and its difficulty in handling dictate that laboratory samples be of small size, that is of the order of 100 to 200 mg in weight. If the sorbed quantity is in the range 1 to 10% (often the case), then the incremental weight is ! to 20 mg, an amount that is easily de- tected with an analytical balance. For best results the hair should always be weighed directly, not in a container. A suitable configuration is achieved by winding the 100 mg strand around one's finger and stuffing it into a ! oz vial. Enough distilled water is added to wet the hair and the vial is left open overnight in a 50øC oven. By the next day, after drying, a well shaped curl has formed which retains its configuration and can easily be removed by forceps and placed on the balance pan. Hair is very hygroscopic, a fact which creates a problem in gravimetric work. Thoroughly dried hair will gain several percent of its weight in moisture when it is exposed to the atmosphere for only a few minutes. It is thus extremely important to make sure that the sample being weighed is always at the same reference state relative to water vapor. For example, this could be a "bone dry" condition such as is achieved by drying over P2Os or at high temperatures. However, drying by desiccants is time- consuming, while high-temperature drying tends to alter the hair protein irreversibly. On the other hand, simple equilibration for some time at ambient atmosphere is quite unsatisfactory, owing to changes of relative humidity. As a compromise, oven drying at 50øC was adopted. This is low enough so that no damage seems to occur to the hair. The amount of time required to reach equilibrium water content is of the order of 8 to ! 2 hr. Thus samples can conveniently be left overnight in the oven before weighing. (A 10 min period in a desiccator to cool the sample is advisable between the oven and balance.) The reproducibility achieved in this way has generally been very satisfactory. For example, two samples of bleached hair were conditioned in a 50øC oven for a day. Their weights were recorded as 104.8 and 123.4 mg. The samples were then placed in distilled water for three days, removed and dried in the oven overnight. The following day their weights were, respectively, ! 05.0 and 123.6 mg. For accuracy, the hair samples should be dried and weighed several times, both initially and after sorption, until constant values are obtained. In actual practice it was usually found that the weight after the first drying does not change appreciably upon further drying. It is always advisable to run several "controls" for each sorption experiment these are hair samples which are exposed to distilled water for the same time as the du- ration of the sorption. Normally the controls will return to their initial weight but in some cases systematic (i.e., more than one sample) deviations of 0.5 to ! mg can occur. This is possibly related to large changes of ambient laboratory humidity. Such devia- tions should be taken into account for the sorption samples. Some discussion about rinsing procedure is in order here. To a certain extent the method followed must be adapted to the material which is sorbed. Thus, for a cationic polymer, which is very tenaciously sorbed, the hair should be thoroughly rinsed several times. The polymer sorbed will not come off easily even with vigorous washings and more important, the viscous polymer solution which is easily entrapped in the hair must be removed or erratic results will be recorded. At the other end of the spectrum are substances like salts (see below for results on NaBr). In this case sorption is very weak and thorough rinsing completely removes the salt a different procedure must be
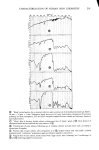
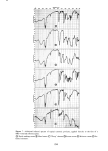
326 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS 1.6 1.4 .4 .2 o ß RADIOTRACER DATA, Na 22 BY STAM & WHITE (1954) /x GRAVlMETRIC, (1976) ß1 I I I I I 0 1 2 3 CONCENTRATION (M) Figure 1. Sorption of sodium bromide by blonde hair followed, which consists in simply patting dry the hair fibers with tissue. This effec- tively removes entrapped liquid. The weight of a tress treated in this way is surprisingly reproducible and corresponds to a kind of "fully hydrated" state. The anionic surfactant sodium lauryl sulfate (also treated below) corresponds to an intermediate case. The surfactant is fairly tightly bound by the hair substrate and is located internally as well as on the surface. However, upon very thorough washing (15 to 30 min) a third or more of it will be desorbed. Hence a compromise protocol is advisable, such as two or three successive short rinses in distilled water to remove adhering liquid and foam. With suitable care the gravimetric procedure outlined above has yielded results which are quite close to those obtained by radiotracers, as shown in the following cases: Sodium bromide, NaBr. Stam and White (9) have reported on the uptake of NaBr by undamaged blonde hair from aqueous solution, using a Na '•2 tag. Their results for several concentrations are given in Figure 1 and show a linear relation between sorp- tion and concentration. Our gravimetric data (also with undamaged blonde hair) done in duplicate at two concentrations are plotted in the same figure. The agreement is surprisingly good, considering that the hair samples are completely different. Note that
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)