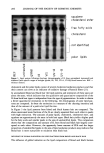
J. Soc. Cosmet. Chem., 47, 185-200 (July/August 1996) Binding of surfactants to stratum corneum K. P. ANANTHAPADMANABHAN, K. K. YU, C. L. MEYERS, AND M.P. ARONSON, Unilever Research U.S., Edgewater, N.J. 07020, Accepted for publication August 15, 1996. Synopsis The binding of surfactants to stratum corneum proteins has been implicated as one of the factors governing their harshness towards skin. In this paper, the binding characteristics of several anionic surfactants, including relatively harsh surfactants such as sodium dodecyl sulfate, TEA-sodium laurate, TEA-sodium oleate, and a relatively milder one such as sodium lauroyl isethionate (SLI), are presented. The effect of variables relevant to cleansing such as solution pH, temperature, and contact time on surfactant binding has been determined. The binding of laurate, oleate, and SDS is significantly higher than that of SLI. The extent of binding correlates with their expected irritation potential measured by the zein solubilization technique as well as with the published results of irritation to skin of cleansing bars based on these surfactants. The reasons for the increased binding of surfactants above their CMC are examined. It is also shown that SLI binding to skin is dependent on the solution pH, exhibiting a minimum in binding in the pH 7 to 9 region. INTRODUCTION It is well established that sodium dodecyl sulfate (SDS) and soaps such as sodium laurate are harsh towards skin (1-5). The harshness of anionic surfactants with compact head groups has been suggested to be due to their ability to bind to stratum corneum, especially stratum corneum proteins (3-5). It is also known that harsh surfactants such as SDS denature water-soluble proteins such as BSA and lysozyme (2,6-11) and solu- bilize water-insoluble proteins such as zein (7). In fact, the harshness of surfactants towards skin has been correlated with their ability to solubilize zein (8,12). Sodium cocoyl isethionate is the main ingredient in several widely used personal wash- ing bars that are known to be significantly milder to skin than SDS and soap (13,14). The mildness of isethionate-based bars is believed to be due, among other things, to the overall composition of the bar as well as the relatively mild nature of its anionic surfactants. Sodium lauroyl isethionate (SLI) is the main component of sodium cocoyl isethionate. Several factors could be suggested to contribute to the relative mildness of SLI. These include its lower critical micelie concentration (CMC) compared to SDS and its lower tendency to bind to stratum corneum because of its larger head group. The binding behavior of SDS to soluble proteins such as BSA and lysozyme has been 185
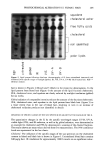
186 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS studied extensively (15-18). Figure 1 represents a typical binding isotherm of SDS to a water-soluble protein (9,17). In the initial region (a), there exists some binding to specific sites on the protein. This is followed by either a plateau or a slow rising part of the isotherm (b) and beyond it a third region corresponding to a massive increase in binding because of cooperative interactions (c). The unfolding of the protein occurs in the cooperative binding region. Beyond the saturation point, the binding isotherm shows a plateau (d), suggesting that further binding of the surfactant does not occur on the protein. Under saturation binding conditions, i gram of the protein can be expected to bind as much as 1.5 to 2 grams of the surfactant (7). It is important to note that all of this behavior occurs below the normal CMC of the surfactant. Some data exist on the binding of SDS to stratum corneum and keratinous proteins (19-28). The main difference between surfactant binding to a soluble protein versus an insoluble protein is that for an insoluble protein, the cooperative binding appears to occur at surfactant concentrations above the CMC. The limited work available on stratum corneum also indicates that the binding of SDS continues to increase above the CMC, although with a slope lower than that in the pre-CMC region. This was inter- preted by Faucher et al. (23) to be due to the restricted diffusion of micelles into the stratum corneum compared to monomers. The reasons for the increase in the binding above CMC itself was not discussed in terms of the thermodynamic activity of the monomer, which does not increase above the CMC. Furthermore, the data does not adequately cover the low concentration region in this case. Although alkyl isethionate-type surfactants are of considerable practical importance in mild cleansing products, their binding behavior to skin has not been investigated in the past. This paper reports the results of binding studies of soaps, SDS, and SLI to human and porcine stratum corneum as a function of relevant variables such as surfactant concentration, solution pH, time of contact, and temperature. (d) log ([el) Figure 1. Schematic plot of the number of bound ligands per protein molecule (n) as a function of the logarithm of the free ligand concentration (c). Region a, specific binding region b, non-cooperative binding region c, cooperative binding, region d, saturation. Reproduced from M.N. Jones, Biochem. J., 151, 109-114 (1975).
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)