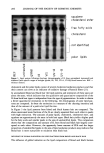
PREPRINTS OF THE 1996 ANNUAL SCIENTIFIC MEETING 285 evaporation process of a colloidal-based consumer product. The vapor pressures of the fragrances phenyl alcohol (PEA) and phenethyl acetate (PEAc) are presented in the different phases found in a system of water, the fragrance, and commercial grade laureth-4. The results are interpreted in the form of molecular interactions described by solubility parameters. METHODS Phase diagrams were determined by visual observation, and liquid crystalline phases were identified by light microscopy with cross polarizers. Low angle x-ray diffraction was used to determine the location of the fragrance molecule in the liquid crystal. Vapor pressures were determined by capillary gc (splitless injector, 5% phenyl substituted methylpolysiloxane column flame ionization detector). Samples of laureth-4, phenethyl alcohol, or phenethyl acetate and water were mixed and equilibrated overnight. Head- space vials were prepared and equilibrated two days before analysis (1-3). In addition, phenethyl acetate was also evaluated in solutions of tetraethylene glycol, decane, and homogeneous tetraethylene glycol n-dodecyl ether. RESULTS AND DISCUSSION Vapor pressure variations of phenethyl acetate in the solvents decane, tetraethylene glycol, benzene, tetraethylene glycol dodecyl ether, and commercial grade laureth-4 are initially less than ideal solutions at high concentrations and greater than ideal solutions at low concentrations (Figure 1). The difference in solubility parameters of phenethyl acetate in the various solvents follows that of the Henry's constants. When phenethyl acetate is added to water, where it has very low solubility, a great !.0- 0.8 0.6 0.2, Y Y ooo x •....- o o.o- 0.8 1.o. Figure 1. Phenethyl acetate vapor pressure in different solvents. Po, is the vapor pressure of pure phenethyl acetate. ', Data points PEAc/Cx2/EO) solution. C), Data points for PEAc/laureth 4 solution. ', Data points for PEAc/benzene solution. •', Data points for •EAc/decane solution. x, Data points for PEAc/ tetraethylene glycol solution.
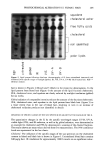
286 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS 1.0 0.$ 0.6 0.4' 0.2 ÷ 0.0. 0.0 I " ' ! " I 012 0.4 0.8 Mole F•ctlon of F• Figure 2. The vapor pressure of fragrances in laureth 4 solutions. I, PEAc. C), PEA. increase in vapor pressure relative to ideal behavior is observed until the solution is saturated at mole fraction - 1.43 X 10 -4. Vapor pressure was somewhat lower than an ideal solution when surfactant was added to phenethyl acetate until the mole fraction reached 0.8, thereafter becoming ideal (Figure 2). When a water-saturated solution was used, the vapor pressure varied with mole fraction by becoming lower than ideal solution at high fragrance concentrations and then becoming higher when the mole fraction of surfactant exceeded 0.45 (Figure 3). This is due to the fact the surfactant is in its monomeric form when the concentration is low, and at high concentrations the polar headgroups begin associating (4,5) with inverse micelies forming when water is present, such as in the saturated solution. After self-association of the surfactant occurs, a higher 0.8 0.6 0.2 0.0 o.0 0.2 0.4 0.6 018 Mole Fr•on of Fragr-aa• Figure 3. The vapor pressure of fragrances in fragrance/laureth 4 solutions saturated with water. The mole fraction is calculated on the non-aqueous compounds only. The value of Po is that of the fragrance saturated with water. I, PEAc. C), PEA.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)