PREPRINTS OF THE 1996 ANNUAL SCIENTIFIC MEETING 287 vapor pressure is seen because the polar groups are unavailable, and the only interactions that the fragrance molecule can experience are with the hydrocarbon chains of the surfactant, similar to the case of fragrance solubilized in decane. This also is the case for the water-saturated solution with the highest vapor pressure occurring at the point of maximum water solubilization. Low-angle x-ray diffraction shows that the fragrance molecule is located near the inter- face with the polar groups and the hydrocarbon chains and not deep within the bilayer. Its presence also influences the partitioning of water in the structure, and the Henry's constant is increased dramatically when the structure changes from liquid (1.5) to liquid crystal (12.3) (2). When comparing phenethyl acetate to phenethyl alcohol where the hydroxyl function- ality can participate, the difference in the surfactant solution is small, showing that the hydroxyl group plays only a minor role in the total molecular interaction in the water poor part of the system. In water, however, it is much more significant, and the Henry's constant is twice that for phenethyl acetate as hydrogen bonding becomes more impor- tant (3). REFERENCES (1) V. D. Roe, M. J. Lacy, and J. D. Stuart, Anal. Chem., 61, 2584 (1989). (2) S. E. Friberg, L. Fei, and P. A. Aikens, J. Mol. Liquids, submitted for publication. (3) S. E. Friberg, T. Huang, L. Fei, S. A. Vona, and P. A. Aikens, Prog. Colloid Polymer Sci., in press. (4) H. Christenson and S. E. Friberg, J. Colloid Interface Sci., 75, 276 (1980). (5) H. Christenson and S. E. Friberg, J. Phys. Chem., 84, 3633 (1980). Delivery of multi-enzyme complexes to the skin surface DAVID WATKINS, MARK ASSMANN, JENEEN WAGNER, CHARLOTTE ZHUANG, PATRICIA SEYMOUR, and JAMES HAYWARD, Col/aborative Laboratories Inc., 3 Technology Drive, East Setauket, NY 11733. Exfoliation of the dead layers of the stratum corneum in order to remove rough patches and to promote the growth of new, healthy skin is an excellent method of reducing the signs of aging. Currently products containing alpha-hydroxy acids (AHAs) are the mainstay of this treatment method. Although AHAs work well for this purpose, the low pH necessary for AHA action tends to be irritating, especially to sensitive skin, and is incompatible with many standard creams and emulsions. The search for an alternative to AHAs that avoid these problems has led us to investigate the use of enzymes as exfoliants. We examined the ability of a proteolytic enzyme to act as an exfoliant by measuring the rate of removal of the stratum corneum from human volunteers. Small patches of the volar forearm were stained for 24 hours with dihydroxy acetone, the active ingredient in
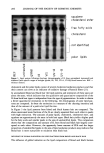
288 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS many self-tanning products. Test materials were then added daily to the same area, and the color of the patch was measured with a Minolta © chromometer. The data in Figure 1 show that a fruit-derived proteolytic enzyme, even at a concentration of only 0.1%, exfoliates much faster than an OTC lotion containing 10% AHA. In addition to proteolytic enzymes, other enzymes, such as those with glycolytic and lipolytic activities, will likely be useful in skin care products. A major difficulty in using proteolytic enzymes in a multienzyme product is that since all enzymes are proteins, they are susceptible to digestion by proteolytic enzymes. Clearly simple admixtures of proteolytic enzymes and other active proteins will have a very limited shelf-life. We have developed a means of sequestering non-proteolytic enzymes away from proteolytic en- zymes in a single-phase, water-based product. Catezomes TM (patent pending) are non- phospholipid, substantive liposomes that we have recently developed. We have used Catezomes TM to encapsulate non-proteolytic enzymes in order to protect them from proteolytic attack. The non-proteolytic enzyme is first encapsulated within the Cate- zomes TM (no attempt is made to remove the unencapsulated material), and then the proteolytic enzyme is added to the external water phase. The unencapsulated non- proteolytic enzyme is degraded by the proteolytic enzyme, and hence the activity of the overall preparation decreases with time (Figure 2). However, the encapsulated non- proteolytic enzyme is protected from attack by the Catezomes T• membrane. Thus, in the encapsulated system, the non-proteolytic activity remains, where, in the absence of Catezomes TM , the non-proteolytic enzyme is completely destroyed (Figure 2). The crux of this invention is the encapsulation of non-proteolytic enzymes within Catezomes TM and the sequestering of proteolytic enzymes on the outside of the lipo- somes. The enzymes on the inside of the liposomes are thus protected from proteolytic A [] 0.1% Enzyme A 10% AHA lotion i i i i 3 4 5 6 Day Figure 1. The rate of exfoliation measured on the human volar forearm.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)