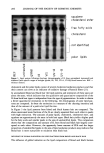
PREPRINTS OF THE 1996 ANNUAL SCIENTIFIC MEETING 269 mg Octyl Palmitate1100g pigment Untreated Lauroyl Lecflh*n Metal Melh•cone Sdane Unreacted Lysme Soap Pedluoro Compound Mica (15 pm) Figure 3. Oil absorption. The high oil absorption of the methicone and the perfluoro compound-treated mica (Figure 3), indicates resistance to wetting by oil, a property that, even more than hydrophobicity, imparts long-wearing attributes to color cosmetics. Conversely, micas coated with lecithin, metal soap, and silane have low oil absorption numbers, indicating that they are easily wet by the oil. Treatments that maximize pigment wetting are preferred for dispersed systems in which uniform pigment wetting or the incorporation of high pigment loads is required. In emulsions in which the pigment is incorporated into the oil phase, migration of the pigment into the water phase and destabilization of the emulsion can be avoided by using treated pigments having a high affinity for the oil phase. Cream powder non-solvent anhydrous systems can be prepared by optimizing pigment wetting with an appropriate surface treatment to maximize the pigment load. Other tests evaluating the effects of the different surface treatments on pigment per- formance in actual products during wear are used as a further means to determine the treatment of choice. REFERENCES (1) E. B. Faulkner and W. J. Zavadoski, Cosmet. Toiletr., 25, 69 (1994). (2) M. L. Schlossrnan, Cosmet. Toiletr., 105, 53 (1990). A novel form of silica: Silica shells CAROL A. S. BREVETT and HOWARD W. JACOBSON, DuPont Specialty Chemicals, Deepwater, NJ 08023. INTRODUCTION A new form of amorphous silica has been developed, the silica shell. These shells (called Zelec © SIL) are hollow ellipsoids of porous silica. They are prepared by a patented
270 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS DuPont process whereby a calcium carbonate particle is coated with silica. The carbon- ate is then leached out, leaving only the outer silica coating, or shell, which is about 0.04 to 0.08 microns thick. The silica shell particles average 3 microns in diameter. Due to their morphology and porous nature, they have a large surface area (about 100 m2/g) and high oil absorption (600 g of 70 cSt mineral oil per 100 g of powder). These silica shells may be thought of as "micron-sized silica sponges." The hollow structure and porosity of the shells allow them to scatter small amounts of light. The shells are a white powder, which gives a white color to formulations, but it is transparent when rubbed onto the skin. In addition, the shells have a smooth, silky feel. As a component in cosmetic formulations, the shells blend easily into either the water or oil phase, producing a stable emulsion that will not whiten the skin. The shells may be used over a wide range of processing temperatures, from 0 to 120øC. Building on this technology, the shells can be coated with metal oxides such as titania and iron oxide hydroxide (FeOOH) or with metals such as silver. Like the shells, the various coated shells also disperse into oil and water very easily, and feel smooth when rubbed into the skin. The shells and titania-coated shells are white, but the color of the FeOOH-coated shells is orange to brown, depending on the amount of FeOOH. The coated shells have a somewhat lower mineral oil absorption of 350 g oil per 100 g of powder. The coated shells may be used as concealers (line blurring) and color enhancers, and may help to boost SPF. APPLICATIONS THICKENING AGENT Dispersions can be made in oil or water, although the shells will hard-pack in water if left overnight. High viscosities can be achieved with the shells in 70 cSt mineral oil these dispersions are shear thinning (see Figure 1). In dimethicone (Dow Corning 200 Fluid, 100 cSt) the shells produce a viscosity that is independent of the shear-rate (see Figure 2). RETENTION OF MATERIALS INSIDE THE SHELL STRUCTURE The high oil absorption value of the shells is a sum of three locations of absorption: within the pores of the shell wall, within the interior of the hollow ellipsoids, and between the individual particles. The volume of the pores in the shell wall, which range from 17 to 3000 Angstroms, is 0.2 cc/gram, as measured by nitrogen gas adsorption. The volume of the hollow ellipsoids is about 3 cc/gram (calculated). The volume of liquid absorbed to make a paste is 7 to 8 cc/gram hence the volume of liquid between the individual particles, by difference, is 3 to 4 cc/gram. The shells are fairly nonselective micron-sized sponges. They absorb water, ethanol, isopropanol, organic fluids such as mineral oil and silicones, and perfluorinated organic fluids such as polyperfluoroisopropyl ether (PPIE) (Fluortress © L-16). When a paste of the shells with PPIE is dispersed into water, most of the PPIE separates out of the shells. The shells remain in the aqueous phase, and •9F NMR shows that some PPIE is still present in the shells. This shows that small amounts of some organic materials may be retained in the shell structure.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)