PREPRINTS OF THE 1996 ANNUAL SCIENTIFIC MEETING 279 Figure 1. Multiple emulsion schematic. Oil Phase (incl. emulsifier) Walcr Phase •'• Dinpict '• Intcffacc Figure 2. Droplet retention time method. such as vitamins and enzymes can frequently be dissolved in them without compromis- ing biological activity or stability. To encapsulate actives in multiple emulsions, a three-step procedure is necessary. First the internal w/o emulsion containing the active in the aqueous or polyol phase must be prepared. In parallel, the external aqueous phase must be prepared. Then the two must be blended. The external aqueous phase must be formulated with a rheology that ß shows excellent esthetics on the skin ß minimizes rupture and loss of encapsulated active during the blending process ß effectively stabilizes multiple droplets against creaming or coalescence once dispersed Additionally, no micelles must be present that could migrate across the internal inter- faces. These requirements can generally be met by a well-balanced gel network struc- ture, which will allow the incorporation of nearly any ratio between 1 and 30 wt% of the primary w/o or p/o emulsion. EXPERIMENTS We were able to prepare a polyol-in-oil emulsion in which vitamin C, as ascorbic acid,
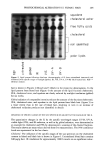
280 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS was dissolved in propylene glycol at 10 wt% to form the internal polyol phase, and cyclomethicone formed the oil phase. Twenty wt% of the p/o emulsion was then dispersed into an aqueous phase containing a well balanced liquid crystalline gel net- work structure, in this case based on Nikkomulese 41TM (polyglyceryl-10 pentasterate (and) behenyl alcohol (and) sodium stearoyl lactylate). See Figure 3. To check chemical stability of the absorbic acid, its concentration in the system was measured by HPLC after storage at 40øC for six weeks. We incorporated 1 wt% of NaCI in the propylene glycol phase as a means to detect leakage between the internal phase and external phase via conductivity measurements (2). Conductivity readings were taken during and directly after manufacturing (Figure 4), and after storage for six weeks at 25 and 40øC. Droplet evolution was also observed under the microscope. From HPLC, 99.5% of the originally incorporated ascorbic acid was determined still to be present after six weeks at 40øC. From microscopy, the size distribution of the dispersed phase reaches steady state after about 50 to 60 seconds. From conductivity measurements, it could be observed that droplet break-up during the initial dispersion of the p/o emulsion into the external aqueous phase released 34 + 5% of the ascorbic acid after this time period. No further release could be observed in the sample stored for six weeks at 25øC. At storage temperatures of 40øC after six weeks, the conductivity of the system was increasing slightly. A release of an additional 10 + 5% was calculated. At least 50% of the encapsulated ascorbic acid could probably be incorporated in a stable fashion for a significant period. Under the microscope, the multiple droplets appeared to be stable, even over six months at 40øC. This formulation, containing overall 2% of ascorbic acid, was SPF-tested on five panel members against the same vehicle without the vitamin C and against Nivea cream. None of the formulations contained a UV filter. The tests indicated an SPF value of 1 for the vehicle and for the Nivea, but an SPF of 5 (COLIPA) for the formulation containing the ascorbic acid, indicating some effectiveness of stabilization and delivery. .• '•, -:' . --. '• - '-'--•:& • .: '.--a- •-'a•. ' •'•-I.' ':' •'= • ".,--" .•'•- ß •.,- ,.•-:'•.r- -: -• ' '• • .•: -• •, •:- • •,=.• . ..• ...... • .•. Figure 3. Polyol-in-oil-in-water droplet (400x) after 4 weeks at 40øC
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)