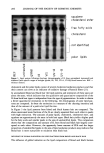
214 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS reactions (3-11). In 1958, Sch/Sberl and Griifje (10) showed that the first displacement reaction (Eq. 1) leads to the formation of the mixed disulfide of keratin and mercaptan. Later, in 1974, Bor6 and Arnaud (11) confirmed that nucleophilic substitution occurs in the second displacement reaction (Eq. 2). Ker-S-S-Ker + RS-H •-• Ker-S-S-R -3- Ker-S-H (1) keratin reduced keratin (cystine) mercaptan mixed disulfide (cysteine) Ker-S-S-R + RS-H--• R-S-S-R + Ker-S-H (2) reduced keratin mixeddisulfide mercaptan disulfide (cysteine) Ker - S - S - Ker NH 3 + -- CH2 -- Ker -- S -- S -- CH2 -- CH2 -- Ker - S - H keratin -}- • -}- (cystine) CH2 -- SH CH2 -- NH3 + reduced keratin (cysteine) cysteamine mixed disulfide with cysteamine (3) Recent studies have shown that a variety of other compounds may result from the interaction of mercaptan with keratin. Amino acid analysis has been used to identify residues of lanthionine, lysinoalanine, mixed disulfides, cleaved fatty acid fragments, and even small changes in the protein and lipid composition of permanently waved hair (12-20). In this study, the kinetics of reduction of hair by cysteamine and ammonium thiogly- colate (ATG) were investigated using amino acid analysis and single-fiber tensile ki- netics (SFTK). Both measurements have been previously used to study disulfide reduc- tion however, the results of these two methods have yet to be correlated. The results of this study indicate that SFTK and amino acid analysis agree qualitatively but not quantitatively. This discrepancy may result from the fact that SFTK measurements determine the removal of stress-supporting disulfide bonds, while amino acid analysis determines the removal of all disulfide bonds in the fiber. Cysteamine (NH 3 +-CH2-CH2-SH) has an ammonium group adjacent to the thiol. The ammonium group may directly affect the properties exhibited by cysteamine as a re- ducing agent for permanent waving. Once cysteamine cleaves the keratin disulfide when the attached amino group is protonated (Eq. 3), ionic crosslinks may be formed with carboxylic acid residues in the keratin fibers. This will result in the replacement of a covalent crosslink with an ionic crosslink that is not completely cleaved until the second replacement reaction occurs (Eq. 2). Therefore, the effects of reduction on the physical and chemical properties were studied and compared with the effects produced by am- monium thioglycolate, a reducing agent that has been extensively studied in previous work (4,16,17,21-25). EXPERIMENTAL MATERIALS Medium-brown, virgin hair from a single source was obtained from DeMeo Brothers (New York). This hair sample was used for all the studies described in this report. The reducing agents ammonium thioglycolate and 2-aminoethanethiol HCI (cysteamine)
REDUCTION OF HUMAN HAIR 215 were obtained from Evans Chemetics (Waterloo, NY) and Aldrich Chemicals (Milwau- kee, WI), respectively. Amino acid standard kits, 0.4 N borate buffer, dithiodipropi- onic acid, and OPA reagent were obtained from Hewlett-Packard (Piscataway, NJ). J.T. Baker HPLC-grade acetonitrile, ammonium hydroxide (30%), glacial acetic acid, iso- propyl alcohol, methanol, sodium acetate trihydrate, and sodium hydroxide were ob- tained from VWR Scientific (Piscataway, NJ). Iodoacetic acid and phenol were obtained from Aldrich Chemicals (Milwaukee, WI). Hydrochloric acid (0.1 N) was obtained from Fisher Scientific (Pittsburgh, PA). Hydrochloric acid (6 N, constant boiling), tetrahy- drofuran (silylation grade), and triethylamine (sequanal grade) were obtained from Pierce Chemicals (Rockford, IL). Liquid nitrogen and argon gas were obtained from JWS Technologies (Oakland, NJ). Deionized water used in all experiments was obtained using a Millipore system. EQUIPMENT A Waters Pico-Tag Work Station © was used for hydrolysis of the hair samples. An automated Hewlett-Packard Series II 1090 liquid chromatograph with photodiode array detector was used for analysis of the samples. The instrument was connected to a Hewlett-Packard 9000 Pascal Chemical Station ©, which ran the AminoQuant II soft- ware. An Amino Quant II © 200-cm x 2.1-mm i.d. 5-p•m particle size ODS Hypersil (C•8) column (Hewlett-Packard) with a 20-cm x 2.1-mm i.d. 5-p•m particle size guard column (Hewlett-Packard) was used for separation. The column temperature was set at 40øC, and a flow rate of 0.450 ml/min was used to deliver a gradient. Stress-relaxation and stress-strain measurements were made on the Miniature Tensile Tester (Dia-stron). Temperature was controlled by a Techne © Model 1252-00 circulating waterbath. SFTK METHODOLOGY Reducing solutions. Simple reducing solutions containing only the mercaptan (ATG or cysteamine), Millipore water, and ammonium hydroxide to adjust pH were prepared to produce 1 M solutions having pHs between 7.0 and 9.5. These solutions were used for both amino acid analysis and SFTK measurements. Hair sample preparation. The hair fibers were shampooed with a 10% (w/w) solution of sodium lauryl sulfate in Millipore water, rinsed thoroughly, and allowed to dry. SFTK method. Single-fiber tensile kinetics (SFTK) is a method for investigating reduc- tion kinetics of hair using single fibers based on stress relaxation caused by disulfide bond cleavage (1). The data obtained from SFTK measurements are used to elucidate information about the rates and mechanisms of reactions of reducing agents and to derive mathematical models (1). For purposes of this study utilizing the Miniature Tensile Tester, the SFTK method was modified as described previously (26,27) in order to study the effects of reduction by ATG and cysteamine at different pHs. The data obtained from stress-relaxation measurements were used to determine the reaction rate constants (k). Fiber selection test. Hair fibers were preselected by straining the untreated fibers into the yield region in water ((25% extension) and measuring the force necessary to extend the fiber (5-7,22-25,28-31). Each 30-mm fiber segment was allowed to soak in water for
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)