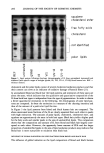
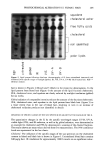
BINDING OF SURFACTANTS 197 binding occurs above the CMC. Obviously the situation is complex, and a quantitative model is required to sort out the details. All of the four factors discussed earlier are in line with the results obtained for the kinetics of SDS binding to human and guinea pig stratum corneum. In both these cases, the binding was found to increase with increase in time up to about six hours. Super- imposed over this increase, there exists a sharply rising region at SDS levels well above the CMC. As mentioned earlier, the relatively mild SLI, on the other hand, does not exhibit such a marked increase in binding above its CMC. The relative magnitudes of different miceliar contributions to enhanced surfactant bind- ing above the CMC are not clear at present. Binding experiments with already-dispersed corneocytes as well as those with barriers that are damaged by techniques such as thermal pretreatment or sonication may provide better insight into the relative contributions. EFFECT OF pH ON SLI BINDING As mentioned earlier, SLI binding was found to exhibit a minimum around pH 7-9, with an increase in both the acidic and alkaline regions. This behavior can be explained in terms of the dependence of properties of the stratum corneum proteins and lipids on pH. An underlying assumption in the following analysis is that "true equilibrium" is difficult to attain in a complex system such as stratum corneum and that the reported binding at different pH values may be at different stages of equilibrium depending upon such factors as thermodynamic binding potential, accessibility to different binding sites, etc. An increase in pH can be expected to decrease the binding of the anionic surfactant to keratin. This is because keratin has isoelectric points at about pH 5 (47). Above this pH, the net negative charge on the protein can be expected to increase with increase in pH. This, in turn, will lower the thermodynamic driving force for surfactant binding to the protein. The experimentally observed decrease in binding with increase in pH up to about a pH of 9.0 is consistent with this expectation. However, the binding appears to increase above pH 9. This is possibly due to the effect of pH on matrix lipids as well as the membrane lipids. In general, the aqueous solubility of the fatty acids, which are a significant component of the bilayer lipids in the stratum corneum (5), can be expected to increase with increase in pH, especially at pH values above 8 or 9. Even though the ionization of the carboxylic acid group can occur at pH values above 5, formation of an acid-soap complex limits the solubility of the soap below pH 9. Above this pH, a marked increase in solubility with pH can be expected. Thus, the delipidization process itself may be more favorable at pH values above 9. Furthermore, the negative charge on the protein structure itself can be expected to increase with increase in pH, and this may reduce the conformational stability of the folded protein. Thus the overall accessibility and vulnerability of the structure to surfactants can be expected to increase eventually with increase in pH. This cutoff seems to be around pH 9 and becomes pronounced at pH values 10. The effect of pH on the mildness of formulated skin cleansing compositions is complex and still a matter of debate (see reference 48 for a review of the subject). For formulations containing potentially ionizable components, Murahata and Aronson recently showed that increase in pH can have a pronounced effect on mildness (48). The above discussion
198 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS and the results in Figure 8 indicate that an alkaline pH can also increase the suscepti- bility of the stratum corneum to increased binding of anionic surfactants, which can in turn lead to protein unfolding. This effect is thus likely to also contribute to the harshness of alkaline compositions to the skin. SUMMARY AND CONCLUSIONS 1. TEA-oleate binds to stratum corneum in higher amounts than all other surfactants at low surfactant concentrations. This is consistent with the expected higher surface activity of longer-chain soaps compared to lower-chain-length soaps and surfactants. At concentrations above about 20 mM (for example, at a concentration of 40 mM), the surfactant binding to HSC is about the same for oleate, laurate, and SDS. Importantly, the binding of SLI is significantly lower than that of the other three surfactants. The extent of binding correlates with the irritation potential of these surfactants measured by the zein dissolution test as well as with the well-known clinical irritation of cleansing bars based on these surfactants. 2. Binding of SDS, TEA/Na oleate, and TEA/Na laurate to HSC increases above the surfactant CMC. Similarly, the binding of SDS to guinea pig stratum corneum shows a sharp increase at concentrations well above CMC. The reasons for the increase in surfactant binding above their CMC are not clear at present. The role of micelles in removing lipids and increasing the accessibility of membrane proteins to surfactant monomers may be implicated in the enhanced surfactant binding at concentrations above the CMC. 3. Increasing the temperature from room temperature to 37øC results in an increase in binding of SDS to HSC, with no measurable effect on SLI. 4. The SLI exhibits a pH-dependent binding behavior to HSC, with a minimum around the pH 7-9 region. REFERENCES (1) C. Prottey, Factors which determine the skin irritation potential of soaps and detergents, J. Soc. Cosmet. Chem., 26, 29-46 (1975). (2) G. Imokawa, K. Sumura, and M. Katsumi, Study on skin roughness caused by surfactants: II. Correlation between protein denaturation and skin roughness, J. Am. Oil. Chem. Soc., 52, 484-489 (1975). (3) G. Imokawa and T. Takeuchi, Surfactants and skin-roughness, Cosmet. Toiletr., 91, 32-46 (1976). (4) L. D. Rhein and F. A. Simion, "Surfactant Interactions with Skin," in Interfacial Phenomena in Biological Systems, Surfactant Science Series, Vol. 39, Max Bender, Ed. (Marcel Dekker, New York, 1991), pp. 33-49. (5) W. Matthies, "Dermatological Observations (Humans)," in Anionic Surfactants, Their Biochemistry, Toxicology and Dermatology, 2nd ed., Surfactant Science Series, Vol. 43, C. Gloxhuber and K. Kun- stler, Eds. (Marcel Dekker, New York, 1992), pp. 291-329. (6) J. Steinhardt and J. A. Reynolds, Multiple Equilibria in Proteins (Academic Press, New York, 1969), pp. 234-301. (7) C. Tanford, The Hydrophobic Effect.' Formation of Micelles and Biological Membranes, 2nd ed. (Wiley- Interscience, New York, 1980), pp. 146-164. (8) M. J. Schwuger and F. G. Bartnik, "Interaction of Anionic Surfactants with Proteins, Enzymes and Membranes," in Anionic Surfactants, Surfactant Science Series, Vol. 10, C. Gloxhuber, Ed. (Marcel Dekker, New York, 1980), pp. 1-49.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)