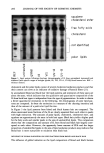
PREPRINTS OF THE 1996 ANNUAL SCIENTIFIC MEETING 277 [ l •%[]-I•'•n I •CN ] o 111v• Figure 2. Effect of cosmetic ingredients on formaldehyde formation. FORMALDEHYDE ./ 70,, / 60, so j./ 40 •-"/ • i a. :30, 20. 10 0- 1 3 9 27 36 0 14 30 49 FORMULA IN DEVELOPMENT FINAL FORMULA Time (In weeks) Figure 3. Stability of DHA degradation in new cosmetic products. types of ingredients used in cosmetic formulations and were able to stabilize dihydroxy- acetone in different types of cosmetic formulations: creams, lotions, mists and sprays (Figure 3). CONCLUSIONS As a result of our investigations and experiments via HPLC and GC/MS, we are pro- posing a mechanism for DHA degradation. Using our findings, we examined and insured the necessary conditions regarding DHA as an incoming raw material and as an ingredient in products. Such technology has resulted in a significant improvement of the stability of Estee Lauder self-tanning products. REFERENCES (1) A. A. Fisher, Contact Dermatitis (Lea & Febiger, Philadelphia, 1986). (2) W. R. Summers, Anal. Chem., 62, 1397-1402 (1990). (3) T. Nash, Biochem. J., 55, 416-421 (1953). (4) A. H. J. Gromping et al., Chromatographia, 35(3/4), 142-148 (1993).
278 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS (5) A. Ostrovskaya et al., Pittsburgh Conference Abstracts, 916 (1996). (6) R. E. Smith, Ion Chromatography Applications (CRC Press, Boca Raton, FL, 1988). (7) D. P. Lee et al., LC-GC, 5, 261-266 (1986). Stabilization and delivery of vitamins and enzymes using multiple emulsions GERD H. DAHMS, IFAC (Institute for Applied Colloidtechnology), Duisberg, Germany. INTRODUCTION The ability to deliver highly specific and complex actives to the skin will become increasingly important in cosmetic and "cosmeceutical" formulations. Many of these actives will not be stable in the traditional water or oil phases of O/W or w/o emulsions. New technologies will be necessary to permit such actives to retain and deliver their biological activity on the skin. Vitamins and enzymes have gained increasing visibility in the area of skin care as antioxidants with desirable activity but often poor stability in cosmetic vehicles. We have applied our experience in the formulation of multiple emulsion systems to develop an approach to the encapsulation and controlled delivery to the skin of such ingredients. FORMULATION OF STABLE MULTIPLE EMULSIONS Multiple emulsions can be defined as X-in-oil-in-Y systems, most commonly water-in- oil-in-water systems (Figure 1). They have a long history of study as systems with significant potential for encapsulation, targeted delivery, and controlled release, but have not been widely adopted because of the traditional difficulty of stabilizing them. We have found that the viscosity and elasticity of the interfacial films seem to be particularly strong predictors of multiple emulsion stability. These parameters deter- mine the coalescence rate in the w/o and w/O/W emulsion. Rigid interfacial layers provide a barrier to coalescence. They also provide a barrier to the phase-transfer of actives. Thus the selection of emulsifiers for their capability to build liquid crystalline structures at both the w/o and O/W interfaces in multiple emulsions has been found to improve stability and encapsulation capability markedly (1). An excellent screening technique for w/o emulsifiers in this regard is the retention time of a water droplet at an oil-water interface (Figure 2). Using this technique, we have been able to select emulsifiers that permit use of a polyol (for instance propylene glycol) as the internal "aqueous" phase in place of water. Polyols have the advantage that actives
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)