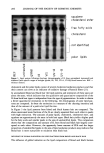
PREPRINTS OF THE 1996 ANNUAL SCIENTIFIC MEETING 271 100000: 10000 1000 100 10 [] 9% ß 7% ß 5% + 3% ß 1% x 0% 0.5 I 10 100 500 Shear Rate (/sec) Figure 1. A plot of viscosity vs shear rate for various loadings of shells in 70 cSt mineral oil. 500O 1oo 0.5 1 m ß 10 100 [] 7%in Isohcxadccanc ß 7% in Cyclomcthicone ß 7% in Dimethicone 5OO Shear Rate (/sec) Figure 2. A plot of viscosity vs shear rate for 7% shells in various fluids. (Cyclomethicone is Dow Corning 345 Fluid dimethicone is Dow Corning 200 Fluid 100 cSt.) Table I Viscosity (cP) of Emulsions Formed With Shells or MAS Oil phase Water phase Viscosity Control--MAS 8500 None None 7800 Shells 8800 Shells 4500 When a paste of shells and mineral oil is dispersed into water, the mineral oil is displaced from the shells. This shows that although the shells are compatible with many types of fluids, they will preferentially absorb water from an oil/water mixture.
272 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS Table II Viscosity (cP) of Emulsions Formed With Shells and Titanium Dioxide Oil phase Water phase Viscosity TiO 2 TiO2, shells TiO 2 Shells Control: no powders shells TiO2, shells TiO 2 8000 7820 8060 8600 9780 8080 Table III Viscosity (cP) of Emulsions Formed With Shells and Titanium Dioxide Control: no powders 8080 Titanium dioxide only 5700 Shells and titanium dioxide 13300 Shells only 15100 IMPACT ON THE VISCOSITY OF AN EMULSION The shells were incorporated into modified versions of a literature formulation (1) in place of the magnesium aluminum silicate (MAS) thickener. The viscosity of the emul- sion was measured at a shear rate of 10/sec. The results in Table I are for emulsions in which the only particulates are shells or MAS. In this case, incorporating the shells into the oil phase of the emulsion lowers the viscosity by 50% compared to the control. The shells were then incorporated into a similar formulation that included particulate ultrafine titanium dioxide. The results are shown in Table II. In this particular series, similar viscosities are achieved regardless of whether the shells are in the oil or water phase. In another variation of this formulation, twice as much particulate material as in Table II was used, and both of the powders were dispersed into the water phase. The results in Table III show that the shells alone increase the viscosity more than any other combination. The combined results of Tables I, II and III show that the viscosity of a formulation may change by a factor of about 3 when the shells are added. ACKNOWLEDGMENTS Sample preparation: Belford Hogate. Viscosity measurements, Brookfield © RVT DVII cone and plate viscometer: Chris Bowers, Ward Gibson. Electron microscopy: Nancy Tassi. REFERENCE (1) J. Woodruff, Cosmetics and Toiletries Worldwide (Aston, 1994), pp. 179-185.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)