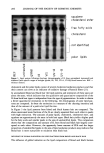
222 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS lOO i • ,,I, ß 8o 6O 40 0 5 10 15 20 25 3o Time (minutes) Figure 3. Comparison of the percentage of cystine reduced in single-head virgin hair by 1 M ammonium thioglycolate, pH 9.4, and 1 M cysteamine, pH 7.6, 23øC, at various time lengths. O, 1 M ATG, pH 9.4 (n = 4) V, 1 M cysteamine, pH 7.6 (n = 4). 0 -7, I I I I 0 200 400 600 800 1000 Time (seconds) Figure 4. SFTK measurements: comparison of the reduction of stress-supporting disulfide bonds in single- head virgin hair by 1 M ammonium thioglycolate, pH 9.4, with 1 M cysteamine, pH 7.6, 23øC, using the mean reaction rate constant for each condition and first half-life. O, 1 M ATG, pH 9.4, slope = --7.44 X 10 -3 s-• ,, 1 M cysteamine, pH 7.6, slope = --3.29 X 10 -3 s -• stress-supporting disulfide bonds by ATG was faster than by cysteamine under condi- tions yielding equal concentrations of RS-. As shown in Figure 6, the results from amino acid analysis correlate well with the results
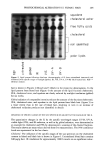
REDUCTION OF HUMAN HAIR 223 0 • -5 Half-life= 123 s Z -6 -- -7 I I I I 0 200 400 600 800 1000 Time (seconds) Figure 5. Amino acid analysis: comparison of the reduction of whole-fiber disulfide bonds in single-head virgin hair by 1 M ammonium thioglycolate, pH 9.4, with 1 M cysteamine, pH 7.6, 23øC, using the mean reaction rate constant for each condition and first half-life. [•, 1 M ATG, pH 9.4, slope = - 5.65 X 10 -3 s-• /•, 1 M cysteamine, pH 7.6, slope = -1.79 X 10 -3 s -• from the SFTK measurements. Both studies indicate that reduction of disulfide bonds by ATG is faster than by cysteamine. The rate of reduction for both treatments was faster from the SFTK measurements, as the hair fibers were reduced under 1.5% strain. The rate constant from SFTK measurements was faster, as only the removal of stress- supporting disulfide bonds is monitored in this measurement. The correlation of results indicates that stress-relaxation data represent not only the removal of stress-supporting disulfide bonds but correlate with disulfide reduction in general. It is possible that the difference in the rates of reaction for ATG and cysteamine may result from the degree of fiber swelling caused by differences in pH of the solutions. The influence of swelling on the condition of the hair has been studied by several authors (2,31,33-38). Hair swelling occurs diametrically and is accompanied by lateral con- traction. The rate and extent of hair swelling is dependent on pH (increasing sharply above pH 9) (5,37) and concentration of the reducing agent (37). However, there is no difference in swelling of hair in the pH range of 8-10 in the absence of reducing agents. Furthermore, Wortman and Souten (39) have shown that length setting does not occur therefore, changes in length from swelling are unlikely. This result indicates that the measured decreases in force can be attributed solely to decreased fiber strength. Thus, it seems likely that rather than "leading to" faster reduction, greater swelling in re- ducing solutions "results from" faster reduction, at least in the pH range of 8-10. Swelling affects both the condition of the hair and the diffusion rate of the reducing agent into the hair. An increase in the degree of swelling causes an increase in the rate of diffusion of the reducing agent and a decrease in the processing time (i.e., rate of reaction). Overall, the percentage of disulfide bonds cleaved is controlled by the pH of the solution and the pK of the reducing agent (34). Therefore, at pH 8.0, cysteamine
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)