J. Soc. Cosmet. Chem., 29, 323-337 (May 1978) Interaction of keratinous substrates with sodium lauryl sulfate.. I. sorption J. A. FAUCHER and E. D. GODDARD Union Carbide Corporation, Tarrytown, NY 1059I. Received September 9, 1977. Presented at Annual Seminar Meeting, Society of Cosmetic Chemists, May I977, Montreal, Canada. Synopsis Use was made of radiotagged SODIUM LAURYL SULFATE (SLS) to determine its sorption by skin and hair. In the initial stages uptake is linear in square root of time, indicative of a diffusion process. The uptakes determined by radiotagged SLS were successfully correlated with data from a simple gravimetric method and showed that the latter procedure can be used satisfactorily under certain conditions when radiotagged com- pounds are not available. The influence of some additives on the SORPTION of SLS was studied. Salt increases the sorption, while nonionic SURFACTANTS (which are not themselves sorbed) substantially depress it. Finally, the relation of the sorbed SLS to water of hydration of KERATIN is examined. It is con- cluded that most, if not all, the sorbed material is bound to keratin, rather than existing in an "internal" solu- tion. INTRODUCTION Surl5•ctants and soaps are known to be irritating to the skin and under extreme condi- tions can have adverse effects on hair. Scientific study of the action of these materials is hampered by a lack of data on their uptake by various keratin substrates. The avail- ability of sodium lauryl sulfate (SLS) in radiotagged form makes it relatively easy to study the kinetics of sorption of this model surfactant over a wide range of concentra- tions and times, and to explore the various effects of additives. There are few data in the literature concerning the sorption of surfactants by human hairß Two studies (1,2) have dealt with long-chain quaternary ammonium halides (typical cationic surfactants), but both were conducted at concentrations well under 1% A brief ra•di•otracer stud was made of sodium ac¾1 sarcosinates (3) in which the ß _ .... r_a_cer•_ st• concentration was as h•. However, n•ot•h_ing ba•s_a_p_.peared dealing with non- ionics, amphoterics or more common anionics such as sodium lauryl sulfate and its ethoxylates nor have concentrations in the range of 10% been investigated, cor- responding to the actual strength of shampoos. The situation is similar for the substrate skin. There is a large, medically oriented litera- ture on the percutaneous absorption of surfactants-particularly anionics like SLS, but the uptake of surfactant by the skin is not usually considered in these works. Two not- 323
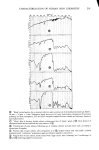
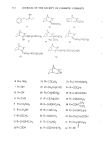
324 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS able exceptions are Harrold and Pethica (4) and Blank and Gould (5). However, both of these papers describe only long times (18 to 24 hr) and low concentrations. Another related study deserves to be cited, namely the work of Garrett (6) on the sorption of surfactants by hide powder. Only low concentrations were employed, but the funda- mental X/t dependence was shown'at short times. It is believed that the present study is the first to examine short times and high surfactant concentrations, corresponding in some degree to normal-use conditions. EXPERIMENTAL Undamaged and bleached hair samples were obtained from DeMeo Bros., New York City, and were used as received. The stratum corneum from neonatal rats was used as a model for human skin. The details of preparation of these membranes have been given in a previous publication (7). This stratum corneum has well developed barrier properties, at least in respect to the transmission of water vapor, as has been shown by Singer and coworkers (8) and confirmed by our own determinations (7). Sodium lauryl sulfate was obtained as a pure white crystalline powder from BDH Chemicals, Ltd., Poole, England. Tagged material was purchased from Amersham/Searle Corp., Arlington Hts., Illinois, in the form of small, individual ampoules. Each ampoule contained 2.47 mg of SLS with an activity of 110 microcuries. The "tag" is present as the S-35 isotope and is thus in the anion of the surfactant: Standapol ES-2 (Henkel Co.)--sodium lauryl ether sulfate with 2 mol of ethylene oxide. Standapol ES-3 (Henkel Co.)--sodium lauryl ether sulfate with 3 mol of ethylene oxide. Standapol 130E (Henkel Co.)--sodium lauryl ether sulfate with 12 mol of ethylene oxide. Tergitol 15-S-9 (Union Carbide Corp.)--the 9 mol ethoxylate of secondary C•a to C •5 alcohol. Solutions of desired concentration were made up of the nonradioactive powder and one ampoule of tagged material was added with stirring. Hair samples of about 100 mg each were placed in 20 ml of the solution for times which varied from a few minutes to 8 hr. They were then removed and rinsed twice for a few seconds to remove entrained solution. For stratum corneum the sample size was approximately 2 mg and the solu- tion was 10 ml. In either case the exposed substrate was dissolved with Unisol (Isolab Inc.) and Unisol-Complement was added. The resulting clear solution was counted by the scintillation method on a Packard 3255 Tri-Carb Spectrometer to determine the amount of SLS sorbed. Triplicate experiments were run and averaged for each experi- mental point on the figures. A GRAVIMETRIC PROCEDURE The availability of accurate sorption data from the radiotracer experiments with SLS described below furnished a benchmark from which a simpler gravimetric technique
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)