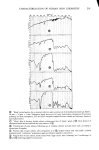
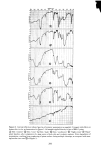
SORPTION OF KERATINOUS SUBSTRATES 335 NO TERGITOL WITH 1% TERGITOL : j ,,/ WITH 5% TERGITOL I I O0 I 2 3 4 5 6 HOURS Figure 10. Effect of a nonionic surfactant on the sorption of sodium lauryl sulfate by bleached hair For undamaged hair the water of hydration amounts to about 35 % by weight of the dry substrate this water is absorbed in less than 1 min. However, the SLS sorption, as shown in Figure 1, goes on for many hours. Furthermore, at low solution concentration of SLS the ultimate amount of surfactant taken up by hair can amount to ten times as much as would be calculated solely from the "external" solution. A study has been made by NMR of the mobility of water in hydrated hair (20). In this work it was found that such water is quite immobile and tightly bound to the keratin. It seems unlikely that SLS can exist as a normal solute in such an environment. The case of stratum corneum is somewhat different in that this substrate absorbs as much as 1000% of its own dry weight over a period of many hours when immersed in aqueous solution. A detailed study of this water (21) shows, however, that much of it is quite restricted in mobility and probably located in the interior of the keratin cells. Again, it seems unlikely that this water ofhydration can behave like the bulk "external" solution in particular, the existence of ordinary micelles therein is improbable because of exclusion effects. Figure 11 shows two curves which compare the actual measured
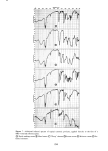
336 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS 60 30 , 2o 10 0 I 2 3 4 5 6 7 8 9 % SLS 10 Figure 11. One hour uptakes of sodium lauryl sulfate in stratum corneum: solid line, as measured experi- mentally dotted line, calculated by assuming that water of hydration has the same composition as the external solution uptakes of SLS at ! hr with the calculated uptakes at each concentration assuming that the water of hydration or "internal" solution has the same concentration as the "external" •olution. The latter curve was calculated from swelling data obtained at a 1 hr exposure for a number of concentrations of SLS. It will be noted that the uptakes at low concentrations (below the CMC) are much greater than the calculated uptakes. But at high concentrations (above 5 %) the calculated uptakes are larger than the measured ones. This lack of agreement clearly shows that the internal solution does not have the same concentration as the external one. It does not exclude the possibility, however, of some SLS monomer existing in free solution inside the stratum corneum. The evidence above suggests that this possible state is unlikely to amount to more than a small frac- tion of the measured uptake. In this connection it may be recalled that collagen and protein in general can bind large amounts of SLS. Nelson (22) has shown that as much as 1.1 to 2.2 g of SLS/g of protein can be bound under the most favorable conditions. Thus the inference that all of the SLS uptake reported here is bound to the keratin is not unreasonable. More light could be shed on this point by a detailed NMR study of the state of the lauryl sulfate anion in hydrated stratum corneum and hair. CONCLUSIONS It has been shown that the uptake of anionic surfactants by hair and stratum corneum membranes is appreciable. With sodium lauryl sulfate, SLS, the uptake increases markedly with concentration even above the critical micelie concentration, and it also
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)