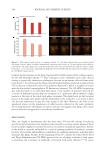
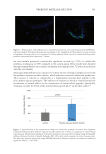
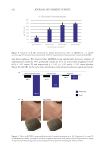
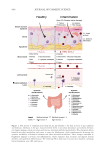
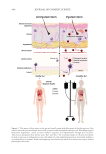
635 Bidirectional Gut-Skin Axis
species and is therefore used for the identification of fungi.12 Both 16S rRNA and ITS
targeted profiling provide high taxonomic resolution for the identification and classification
of bacteria/archaea and fungi, respectively, but their limited scopes necessitate additional
sequencing approaches.12
Conversely, shotgun metagenomic sequencing involves randomly sequencing all the DNA
present in a sample, without any prior amplification or targeting of specific genes. This
enables the cataloguing of genes from organisms within a community, as well as the analysis
of individual genomes within the ecosystem.11 Shotgun metagenomic sequencing has evolved
from utilizing Sanger sequencing to Next Generation Sequencing (NGS), which reduces
costs and increases sequencing depth.13 This process involves fragmenting DNA into smaller
pieces, which requires sophisticated computational systems to reconstruct and align the
sequences to reference genomes.11 Therefore, this method can be limited by the complexity
of data analysis, potential biases in DNA extraction and sequencing, and the need for high
computational resources and robust reference databases for accurate interpretation.11
Long-read sequencing has the advantage of generating long continuous sequences directly
from DNA instead of using amplified fragments and products as sequencing templates,
resulting in more uniform and biologically significant data.14 Examples of this technology
include the competitive Oxford Nanopore Technology™ (ONT) and Pacific Biosciences™
(PacBio), which are useful to sequence highly repetitive genome regions and may
independently be applied for the identification of the entire genome.
Quantitative PCR (qPCR) is an additional molecular technique employed for targeted detection
and quantification within microbiome studies. It involves amplifying and quantifying a
targeted DNA molecule and is, therefore, useful to detect absolute levels of bacteria, viruses,
fungi and protozoa down to the species or strain level, depending on the experimental design.15
Ultimately, metagenomic data is limited in its mechanistic insights and necessitates further
studies into how microbiome functions affect the host. This has led to the integration of other
functional “omics” or “multi-omics” approaches, such as transcriptomics, metabolomics,
proteomics and lipidomics.1
Transcriptomics is the study of the transcriptome, which is the complete set of RNA molecules
expressed by a genome in a specific cell or tissue at a given time. In transcriptomics, qPCR
may also be utilized following RNA sequencing (RNA-seq) to quantify specific microbial
gene expression.1 Alongside metagenomics, which identifies the genetic composition and
functionality of the microbiome, metatranscriptomics provides complementary insights by
revealing which genes are actively expressed by the microorganisms within the community.8
Metabolomics identifies and quantifies metabolites in various environments in order to
understand metabolic changes under different conditions.8 Host metabolomics focuses
on metabolites within the host organism, including compounds like amino acids and
lipids produced by the host’s metabolism. Meanwhile, microbial metabolomics examines
metabolites produced by microbial communities residing in or on the host, such as
antibiotics, toxins, and primary metabolites. Both approaches are crucial for understanding
the interactions between the host and microbial metabolites, and their impact on health
and disease.1 These metabolites are detected using techniques such as mass spectrometry
(MS) or nuclear magnetic resonance (NMR), followed by identification and quantification
by means of chromatography methods, including liquid chromatography (LC) and gas
chromatography (GC).8 This process ultimately provides a practical perspective on
metagenomic analysis and insights into the pathways linked to microbe-host interactions.1
species and is therefore used for the identification of fungi.12 Both 16S rRNA and ITS
targeted profiling provide high taxonomic resolution for the identification and classification
of bacteria/archaea and fungi, respectively, but their limited scopes necessitate additional
sequencing approaches.12
Conversely, shotgun metagenomic sequencing involves randomly sequencing all the DNA
present in a sample, without any prior amplification or targeting of specific genes. This
enables the cataloguing of genes from organisms within a community, as well as the analysis
of individual genomes within the ecosystem.11 Shotgun metagenomic sequencing has evolved
from utilizing Sanger sequencing to Next Generation Sequencing (NGS), which reduces
costs and increases sequencing depth.13 This process involves fragmenting DNA into smaller
pieces, which requires sophisticated computational systems to reconstruct and align the
sequences to reference genomes.11 Therefore, this method can be limited by the complexity
of data analysis, potential biases in DNA extraction and sequencing, and the need for high
computational resources and robust reference databases for accurate interpretation.11
Long-read sequencing has the advantage of generating long continuous sequences directly
from DNA instead of using amplified fragments and products as sequencing templates,
resulting in more uniform and biologically significant data.14 Examples of this technology
include the competitive Oxford Nanopore Technology™ (ONT) and Pacific Biosciences™
(PacBio), which are useful to sequence highly repetitive genome regions and may
independently be applied for the identification of the entire genome.
Quantitative PCR (qPCR) is an additional molecular technique employed for targeted detection
and quantification within microbiome studies. It involves amplifying and quantifying a
targeted DNA molecule and is, therefore, useful to detect absolute levels of bacteria, viruses,
fungi and protozoa down to the species or strain level, depending on the experimental design.15
Ultimately, metagenomic data is limited in its mechanistic insights and necessitates further
studies into how microbiome functions affect the host. This has led to the integration of other
functional “omics” or “multi-omics” approaches, such as transcriptomics, metabolomics,
proteomics and lipidomics.1
Transcriptomics is the study of the transcriptome, which is the complete set of RNA molecules
expressed by a genome in a specific cell or tissue at a given time. In transcriptomics, qPCR
may also be utilized following RNA sequencing (RNA-seq) to quantify specific microbial
gene expression.1 Alongside metagenomics, which identifies the genetic composition and
functionality of the microbiome, metatranscriptomics provides complementary insights by
revealing which genes are actively expressed by the microorganisms within the community.8
Metabolomics identifies and quantifies metabolites in various environments in order to
understand metabolic changes under different conditions.8 Host metabolomics focuses
on metabolites within the host organism, including compounds like amino acids and
lipids produced by the host’s metabolism. Meanwhile, microbial metabolomics examines
metabolites produced by microbial communities residing in or on the host, such as
antibiotics, toxins, and primary metabolites. Both approaches are crucial for understanding
the interactions between the host and microbial metabolites, and their impact on health
and disease.1 These metabolites are detected using techniques such as mass spectrometry
(MS) or nuclear magnetic resonance (NMR), followed by identification and quantification
by means of chromatography methods, including liquid chromatography (LC) and gas
chromatography (GC).8 This process ultimately provides a practical perspective on
metagenomic analysis and insights into the pathways linked to microbe-host interactions.1