637 Bidirectional Gut-Skin Axis
in understanding how these microbial proteins affect host physiological processes and
immune responses. As a result, proteomics provides essential insights into the functional
roles of microbiome-derived proteins and their impact on host health, complementing
the insights gained from metagenomics and metatranscriptomics. Gel electrophoresis,
protein microarrays and MS are all examples of molecular techniques used to investigate
microbiome proteomics.17
Lastly, lipidomics utilizes analytical chemistry tools and principles to study lipid
structures, molecular species abundance, cell functions and, subsequently, microbial-host
interactions.18 This is possible through the identification and quantification of changes in
lipid signaling, metabolism, trafficking and balance within cells.18 MS, NMR and GC are
all approaches used to explore lipidomics in microbiome studies. When used in conjunction,
these multi-omic approaches provide a comprehensive view of microbiome communities,
including both their composition and the functional roles of individual taxa or groups of
taxa within. Integrating this data enables researchers to gain insight into the dynamics of
entire ecosystems, microbial-host interactions and, overall, supports the formulation and
validation of specific hypotheses, aiming for more robust conclusions.8
THE ESTABLISHED ROLE OF THE GUT-SKIN AXIS IN SKIN CONDITIONS
Research has increasingly demonstrated an association between the gut microbiome and
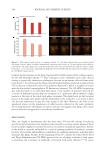
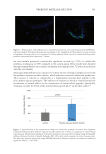
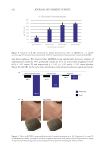
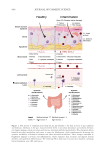
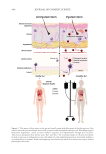
the skin, elucidating the communication pathway known as the gut-skin axis (Figure 1).
The gut microbiome plays a vital role in the regulation of the immune system by protecting
against exogenous pathogens and priming immunoprotective responses. Therefore, it is
responsible for maintaining homeostasis through communication with multiple tissues and
organs, like the skin.25 Consequently, abnormal levels of commensal bacteria in the gut
microbiome may lead to intestinal dysbiosis, which is associated with an altered immune
response and the pathophysiology of multiple inflammatory diseases, related to the gut and
skin, potentially disrupting cutaneous homeostasis.26
The specific mechanisms of communication between the gut microbiome and the skin involve
the immune system and the neuroendocrine system.25 Beginning with diet, part of the healthy
functioning of the gut microbiome includes the degradation of complex polysaccharides into
metabolites, vitamins and SCFAs, the latter of which directly contribute to maintaining the
integrity of the epithelial barrier.27 Conversely, intestinal dysbiosis—which may be caused by
a multitude of factors—is likely to trigger T lymphocyte (T cell) activation and the disruption
of immunosuppressive cytokines and regulatory T lymphocyte (Treg) cells that function to
maintain microbial homeostasis, thereby increasing systemic inflammation.28,29 Oftentimes,
this causes increased intestinal permeability. The disruption of this epithelial barrier may
allow gut microbes, toxins and other metabolites to enter the bloodstream, further triggering
elevated inflammatory cytokine production and T cell responses locally, systemically, and
in the skin.27 In the skin, this may lead to decreased keratin synthesis, altered epidermal
differentiation and, ultimately, the weakening of the skin barrier.28–30
Therefore, microbial metabolites may influence the gut-skin axis by interacting with skin
receptors, potentially affecting the cutaneous environment.3 The most extensively studied
microbial metabolites are SCFAs, which are aliphatic carboxylic acids with fewer than six
carbon atoms, including butyrate, acetate and propionate.31 These are produced by the
fermentation of undigested polysaccharides by intestinal microbes in the colon, primarily
in understanding how these microbial proteins affect host physiological processes and
immune responses. As a result, proteomics provides essential insights into the functional
roles of microbiome-derived proteins and their impact on host health, complementing
the insights gained from metagenomics and metatranscriptomics. Gel electrophoresis,
protein microarrays and MS are all examples of molecular techniques used to investigate
microbiome proteomics.17
Lastly, lipidomics utilizes analytical chemistry tools and principles to study lipid
structures, molecular species abundance, cell functions and, subsequently, microbial-host
interactions.18 This is possible through the identification and quantification of changes in
lipid signaling, metabolism, trafficking and balance within cells.18 MS, NMR and GC are
all approaches used to explore lipidomics in microbiome studies. When used in conjunction,
these multi-omic approaches provide a comprehensive view of microbiome communities,
including both their composition and the functional roles of individual taxa or groups of
taxa within. Integrating this data enables researchers to gain insight into the dynamics of
entire ecosystems, microbial-host interactions and, overall, supports the formulation and
validation of specific hypotheses, aiming for more robust conclusions.8
THE ESTABLISHED ROLE OF THE GUT-SKIN AXIS IN SKIN CONDITIONS
Research has increasingly demonstrated an association between the gut microbiome and
the skin, elucidating the communication pathway known as the gut-skin axis (Figure 1).
The gut microbiome plays a vital role in the regulation of the immune system by protecting
against exogenous pathogens and priming immunoprotective responses. Therefore, it is
responsible for maintaining homeostasis through communication with multiple tissues and
organs, like the skin.25 Consequently, abnormal levels of commensal bacteria in the gut
microbiome may lead to intestinal dysbiosis, which is associated with an altered immune
response and the pathophysiology of multiple inflammatory diseases, related to the gut and
skin, potentially disrupting cutaneous homeostasis.26
The specific mechanisms of communication between the gut microbiome and the skin involve
the immune system and the neuroendocrine system.25 Beginning with diet, part of the healthy
functioning of the gut microbiome includes the degradation of complex polysaccharides into
metabolites, vitamins and SCFAs, the latter of which directly contribute to maintaining the
integrity of the epithelial barrier.27 Conversely, intestinal dysbiosis—which may be caused by
a multitude of factors—is likely to trigger T lymphocyte (T cell) activation and the disruption
of immunosuppressive cytokines and regulatory T lymphocyte (Treg) cells that function to
maintain microbial homeostasis, thereby increasing systemic inflammation.28,29 Oftentimes,
this causes increased intestinal permeability. The disruption of this epithelial barrier may
allow gut microbes, toxins and other metabolites to enter the bloodstream, further triggering
elevated inflammatory cytokine production and T cell responses locally, systemically, and
in the skin.27 In the skin, this may lead to decreased keratin synthesis, altered epidermal
differentiation and, ultimately, the weakening of the skin barrier.28–30
Therefore, microbial metabolites may influence the gut-skin axis by interacting with skin
receptors, potentially affecting the cutaneous environment.3 The most extensively studied
microbial metabolites are SCFAs, which are aliphatic carboxylic acids with fewer than six
carbon atoms, including butyrate, acetate and propionate.31 These are produced by the
fermentation of undigested polysaccharides by intestinal microbes in the colon, primarily