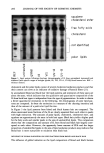
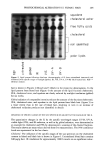
REDUCTION OF HUMAN HAIR 215 were obtained from Evans Chemetics (Waterloo, NY) and Aldrich Chemicals (Milwau- kee, WI), respectively. Amino acid standard kits, 0.4 N borate buffer, dithiodipropi- onic acid, and OPA reagent were obtained from Hewlett-Packard (Piscataway, NJ). J.T. Baker HPLC-grade acetonitrile, ammonium hydroxide (30%), glacial acetic acid, iso- propyl alcohol, methanol, sodium acetate trihydrate, and sodium hydroxide were ob- tained from VWR Scientific (Piscataway, NJ). Iodoacetic acid and phenol were obtained from Aldrich Chemicals (Milwaukee, WI). Hydrochloric acid (0.1 N) was obtained from Fisher Scientific (Pittsburgh, PA). Hydrochloric acid (6 N, constant boiling), tetrahy- drofuran (silylation grade), and triethylamine (sequanal grade) were obtained from Pierce Chemicals (Rockford, IL). Liquid nitrogen and argon gas were obtained from JWS Technologies (Oakland, NJ). Deionized water used in all experiments was obtained using a Millipore system. EQUIPMENT A Waters Pico-Tag Work Station © was used for hydrolysis of the hair samples. An automated Hewlett-Packard Series II 1090 liquid chromatograph with photodiode array detector was used for analysis of the samples. The instrument was connected to a Hewlett-Packard 9000 Pascal Chemical Station ©, which ran the AminoQuant II soft- ware. An Amino Quant II © 200-cm x 2.1-mm i.d. 5-p•m particle size ODS Hypersil (C•8) column (Hewlett-Packard) with a 20-cm x 2.1-mm i.d. 5-p•m particle size guard column (Hewlett-Packard) was used for separation. The column temperature was set at 40øC, and a flow rate of 0.450 ml/min was used to deliver a gradient. Stress-relaxation and stress-strain measurements were made on the Miniature Tensile Tester (Dia-stron). Temperature was controlled by a Techne © Model 1252-00 circulating waterbath. SFTK METHODOLOGY Reducing solutions. Simple reducing solutions containing only the mercaptan (ATG or cysteamine), Millipore water, and ammonium hydroxide to adjust pH were prepared to produce 1 M solutions having pHs between 7.0 and 9.5. These solutions were used for both amino acid analysis and SFTK measurements. Hair sample preparation. The hair fibers were shampooed with a 10% (w/w) solution of sodium lauryl sulfate in Millipore water, rinsed thoroughly, and allowed to dry. SFTK method. Single-fiber tensile kinetics (SFTK) is a method for investigating reduc- tion kinetics of hair using single fibers based on stress relaxation caused by disulfide bond cleavage (1). The data obtained from SFTK measurements are used to elucidate information about the rates and mechanisms of reactions of reducing agents and to derive mathematical models (1). For purposes of this study utilizing the Miniature Tensile Tester, the SFTK method was modified as described previously (26,27) in order to study the effects of reduction by ATG and cysteamine at different pHs. The data obtained from stress-relaxation measurements were used to determine the reaction rate constants (k). Fiber selection test. Hair fibers were preselected by straining the untreated fibers into the yield region in water ((25% extension) and measuring the force necessary to extend the fiber (5-7,22-25,28-31). Each 30-mm fiber segment was allowed to soak in water for
216 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS 60 minutes. The fiber was extended 20% in water and held for five seconds. Only those fibers exhibiting a linear Hookean region and an acceptable Yield region (turnover of 2-5 % and slope approximately equal to 0.1 times the slope in the Hookean region) were used for SFTK measurements. After the untreated hair was measured, it was allowed to re-equilibrate overnight in water to recover its tensile properties. Chemical stress relaxation. When a hair fiber is extended in water by less than 2% of its original length, stress relaxation is complete in approximately 30 minutes. A strain- cycling procedure was used to reduce the time necessary for stress relaxation (1,26,27). The fiber was extended to 2.0% strain in a buffer solution of the same pH and tem- perature as the reducing solution. Following extension, the hair fiber was allowed to stress relax for 60 seconds. The amount of strain was reduced to 1.50% in order to approximate permanent waving conditions, and the fiber was allowed to stress relax until a constant level of stress was achieved (2-3 minutes). After stress relaxation, the buffer solution was replaced rapidly by the reducing solution. SFTK data were collected to monitor the stress relaxation of the disulfide bonds caused by the reducing agent in the hair fiber. The data were collected as grams of force vs time and analyzed using the pseudo first-order kinetics model first described by Reese and Eyring (21). This model has been discussed in detail by Wickett (1,3). The force-vs-time curves follow an exponential decay: F t = F o e -kcøt (4) where F t is the force at time, t, F o is the force at time 0 (before applying the reducing agent), F c is the final force value reached (if reduction is complete, then F c = 0), C O is the concentration of the reducing agent, and k is the pseudo first-order rate constant for the reaction (1,21,26). Rate constants were obtained from the slopes of plots of the natural logarithm of [(F c - Fc)/(F o - Fc)] vs time, which are linear with an intercept of zero when the reaction is truly pseudo first-order. AMINO ACID ANALYSIS METHODOLOGY Mobile phases. A two-solvent system was used with a gradient elution program. Mobile phase A consisted of a 20-mM sodium acetate trihydrate buffer with 0.018% (v/v) triethylamine, and 1.5 ml of tetrahydrofuran adjusted to pH 7.20 + 0.05 with a 1% solution of glacial acetic acid. Mobile phase B consisted of 20% of a 100-mM sodium acetate trihydrate buffer, 40% acetonitrile, and 40% methanol adjusted to pH 7.20 + 0.05 with a 1% solution of glacial acetic acid. Both mobile phases were degassed using continuous helium purging for 15 minutes. Hair sample preparation. Hair from a single individual was extracted with ethanol in a Soxhlet apparatus for 24 hours to remove grease and other materials that may be deposited on the fibers. The extracted hair was soaked in an excess of methanol, rinsed with Millipore water, and allowed to dry. S-carboxymethylation of cysteine. Bundles of hair weighing 200 mg were prepared and reduced for a specified time length (0-30 minutes). Temperature during reduction was held constant at 23øC with a circulating water bath to reduce temperature and concen- tration variation of the reducing solution. Each tress was immersed in an excess (50 ml) of reducing solution. When reduction was complete, the tresses were immediately immersed in a cold 1% solution of iodoacetic acid. This step effectively blocked RS-
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)