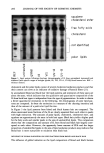
REDUCTION OF HUMAN HAIR 217 groups of the reducing agent and prevented further reduction from occurring by reacting slowly enough to effectively wash off the reducing solution only. The tresses were reacted with a 1% sodium iodoacetate solution, pH = 8.3, for 30 minutes at 75-80øC to form S-carboxymethyl derivatives (SCMC) of all cysteine residues (Eq. 5). The sam- ples were then dried in an oven at 110øC until constant weight was reached. Ker - S-H + I - CH 2 - CO - O-Na + Ker - S - CH 2 - + -• + Na + I- (5) cysteine sodium iodoacetate CO -- O- Na + S- carboxymethyl cysteine Hydrolysis and derivatization. The methodology employed was a modification of the recommended procedure for vapor phase hydrolysis given in the AminoQuant II oper- ator's manual (for a detailed description, refer to reference 27). The samples were hydrolyzed for 24 hours at 110øC with dithiodipropionic acid (DTDPA) added as a protection agent for unreacted cystine, re-dissolved in 0.1 N HCI containing a known amount of an internal standard solution, and filtered with a 0.45-}xm filter. As no residue was left behind after redissolution in 0.1 N HC1, the amino acid content was calculated on the assumption that the hydrolyzed material represented the amino acid composition of the total fiber. The use of DTDPA as a capping agent for cystine was twofold. First, DTDPA protects against the loss of sulfur compounds by oxidation during hydrolysis (32). Second, by converting all non-carboxymethylated cysteine (i.e., mixed disulfide) and cystine to cysteine mercaptopropionic acid (Cysteine-MPA), DT- DPA allows for the separation of cystine from any salts of mercaptan that may remain in the fibers despite the carboxymethylation step (27). DTDPA will react with these by-products and combine with OPA to form isoindole derivatives that may be detected separately from the cysteine-MPA and SCMC derivative (27). This method thereby eliminates the introduction of considerable error into the cystine assay by formation of separate derivatives. However, the percentage of mixed disulfide included in the cys- teine-MPA derivative must be accounted for in the SCMC derivative. Figure 1 shows a sample chromatogram for untreated and treated hair samples. Data collection. The data were collected at a wavelength of 338 nm for the OPA deriv- atives having a bandwidth of 10 nm. A baseline value for total combined cystine and cysteine content in the hair sample was determined by the analysis of an untreated sample. This value was used as a control for the combined value of SCMC and cystine- MPA derivatives isolated in treated hair samples. The area of the SCMC and cystine- MPA peaks was used to determine the increasing amounts of cysteine and decreasing amounts of cystine with increasing reduction time, respectively. The sample concen- tration was detected as picomoles/mg and converted to }xm/g of sample in order to maintain uniformity with previous amino acid studies reported in the literature. RESULTS AND DISCUSSION SFTK MEASUREMENTS A pre-stretch in water or a buffer solution to a constant level of force will effectively break the hydrogen bonding network in the hair ( 1-3,21-25,28,29). Once this network
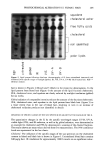
218 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS 1 180 160 140 120 IO0 8O 6O o o •'•1 II ß ¾1 I II II II I I nil ! I I • =zoz _z• - _ (lb) Time [min] I I II II 8O 6O 4O 2O oo[ 80 60 II I I II I I I 2 4 6 8 10 12 14 Time [min] Figure 1. Chromatograms of two single-head hair samples that were vapor phase hydrolyzed and pre- column derivatized with ortho-phthaldehyde, indicating the retention times for the SCMC, cystine-MPA, and residual cystine isoindole derivatives in an untreated hair sample (la) and a hair sample that was treated with 1 M ammonium thioglycolate, pH 9.4, 23øC (lb). has been removed, the assumption that the tensile strength of the fiber is proportional to the number of disulfide bonds remaining may be made (1-3,21). Low strain levels (2%) were chosen to stay in the linear region of the stress-strain curve. By monitoring
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)