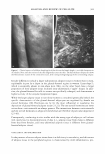
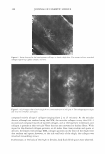
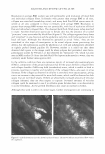
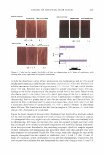
396 JOURNAL OF COSMETIC SCIENCE as well as branched chains of f3-2,l linkages, which vary depending on its origin (1) (Scheme 1). Fructan existing under natural conditions tends to exhibit a structure characterized by the linkage of one molecule of glucose to between dozens and tens of thousands of molecules of fructose. Fructan in plants also exhibits a low molecular weight, with up to 200 fructose linkages, whereas fructan produced by microorganisms tends to have a much higher molecular weight, harboring up to one hundred thousand fructose linkages. The two principal fructan variants are inulin, which contains f3-2,l fructose linkages, and levan, which primarily harbors f3-2,6 fructose linkages as well as different patterns and amounts of branched chains of f3-2,l bonds, depending on its origin. The inulin variant with the shortest linkage is 1-ketose, and the levan with the shortest linkage is 6-ketose. Fifteen percent of flowering plant species produce fructan under natural conditions. In particular, though, fructan is most likely to be found in more highly evolved families, including liliales, poals, astrales, campanulales, palemoniaceae, ericales, dipsacales, bar ley, wheat, and onions (2,3). Currently, one third of the vegetables that exist in the world are considered to harbor fructan. Due to the fact that most species that generate fructan grow in dry, cold areas, fructan is known to function as an osmoprotectant against drought and as a cryoprotectant against cold damage in plants (4,5). A variety of microorganisms, including Pseudomonas sp., Xanthomonas sp., Azotobacter chroococum, Streptococcus salivarius, Bacillus subtilis, Actinomyces, Rothis dentocariosa, Arthro bacter ureafaciens, and Zymomonas mobilis (6-14), are currently known to generate fructan. Fructan generated by microorganisms is primarily of the levan type, which has a higher molecular weight than the fructan synthesized by plants. Levan is known to exhibit a variety of nutritional and pharmaceutical functions, including a hypocholesterolemic effect (15), a promoting effect on the absorption of metallic ions (16), a preventive effect against constipation (17), and antitumor and immunomodulatory effects (18). Considering the variety of properties mentioned above, levan may constitute an appro priate raw material for the formulation of cosmetics and pharmaceutics as well as foods. In this study, we have attempted to assess the cosmeceutical properties of levan gener ated by Zymomonas mobilis and have also tried to evaluate the possibility of its use in the formulation of cosmetics, in order to develop it as a novel cosmetic ingredient. EXPERIMENT AL MATERIALS Purchases of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT), Scheme 1. Structure of levan.
COSMECEUTICAL PROPERTIES OF LEVAN 397 N-(2-hydroxyethyl) piperazine-N' -(2-ethanesulfonic acid) (HEPES), and bovine serum albumin were from the Sigma Chemical Co. (St. Louis, MO). Dulbecco's Modified Eagle's Medium (DMEM), keratinocyte-serum free media (K-SFM), fetal bovine serum, and antibiotics were all purchased from Life Technologies, Inc. (Grand Island, NY). The insert used for the skin equivalent was acquired from Millipore (Bedford, MA). Type I collagen was purchased from Bioland (Bioland, Cheonan, Korea). The ELISA kit, which was used for the interleukin-la release assay, was purchased from Endogen, Inc. (Boston, MA). All of the remaining commercially available chemicals used in this study were reagent-grade, and were used as received. METHODS Preparation of levan from Zymomonas mobilis ( 19 J 20). Zymomonas mobilis ZMl (ATCC10988) was cultured using a 50-liter jar fermentor (KoBiotech Co., Inchon, Korea) with a 10-liter working volume at 30°C, at a pH of 5 .0, for 24 hours. The medium used in this study was composed of 10% sucrose, 1 % yeast extract, and 0.1 % KH2PO4 . After fermentation, the cells were removed from the culture medium, which contained 15 g/1 of levan, via centrifugation (3000 rpm, 10 min). The low-molecular weight by-products, including glucose, sucrose, and oligosaccharides, were then ex tracted via ultrafiltration (0.2-µm filter). The levan was precipitated by the addition of EtOH (EtOH: media = 3: 1 by weight). The levan precipitate was then resuspended in distilled water and settled out repeatedly, twice using the same method. The precipitate was finally dried at 80°C, yielding a powdered product. Analysis of levan structure. In order to characterize the structure of the levan, we recorded the 13C-NMR spectra on a Varian-Mercury Plus 400 spectrometer. The 13C-NMR spectra were then calibrated and reported, using TMS (tetramethylsilane) as an internal standard. The samples were dissolved in D 2 O (about 10 mg/0.5 ml of D 2 O). Our results were then compared with previously reported data (21,22). Molecular weight determination. Molecular weights were determined via gel permeation chromatography, using a Viscotek Tri-SEC system with a Viscotek T-60A dual detector (light scattering, viscometer), under the following conditions: a TOSOH GMPWXL column with a 0.2 M NaCl, 0.1 % NaN 3 aqueous solution eluent, at a 0.5 ml/min flow rate. Poly(ethylene oxide)s were used as standards for the molecular weights. Particle size measurement. The distribution of particle size was determined in water, using an ELS-8000 electrophoretic light-scattering spectrophotometer (Otsuka), a 632.8-nm He-Ne laser (10 mW), at 25°C, with a relative refractive index of 1.3313 (Marquardt analysis method, 100 repeated tests). Transmission electron microscopy. Negative-stain micrographs were prepared on copper grids that had been covered with carbon film. In order to prepare the samples, diluted drops of levan solution were pipetted onto carbon-coated copper grids, then allowed to dry slowly. The copper grids were then stained with 1 % uranyl acetate, and were viewed and photographed using a transmission electron microscope OEM 1010, JEOL, Japan) at an accelerating voltage of 80 kV. Stability test in ethanol. Aqueous levan solution, at a concentration of 5 % on a solid basis, was then mixed with graded concentrations of ethanol (ranging from 10 wt% to 50 wt% of EtOH, in water). Turbidity and precipitation characteristics were observed in order to determine storage stability at room temperature, at 5 °C, and at 40°C, for a total of 45 days.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)