398 JOURNAL OF COSMETIC SCIENCE Measurement of moisturizing effect. Transepidermal water loss and water content were then assessed in order to evaluate the moisturizing effects of levan: (a) Measurement of transepidermal water loss (TEWL) using a Vapometer (23). A group of female volunteers, ranging in age from 22 to 3 7 years, were recruited into this study. Each of these volunteers was then familiarized with the transepidermal water loss (TEWL) method, so as to reduce any emotional stress that might be associated with the testing procedure. Additionally, the volunteers were requested to shave 24 hours prior to the test, and to avoid the intake of any food or drink that contained high levels of stimulatory caffeine. In an attempt to preclude any artificial effects on TEWL resultant from sweating, the volunteers were all instructed to apply an antiperspirant product prior to testing. The TEWL measurements were then conducted in a climate-controlled room, at a temperature between 20° and 25°C, with a relative humidity of 50±5%. Each of the volunteers spent 20 minutes of the equilibration period in the room, relaxing in a prone position with their hands behind their heads, exposing their underarms to the air. TEWL was measured with a Vapometer (Delfin Technologies Ltd.). The TEWL measurements were sequentially recorded at the application sites prior to treatment. These initial measurements were used as pre-treatment control values. Sub sequently, 20 µl of the sample was applied over a 4-cm2 area of the volar forearm (2 x 2 cm), followed by measurements taken at regular intervals for a total of six hours. (b) Measurement of skin moisture content using a Corneometer CM825. A skin hydra tion reading of each sample was recorded with a Corneometer CM825 (Courage Khazaka, West Germany). This equipment consisted of a recording device and an impedance probe that measures electrical conductivity on the surface of the skin. Capacitance refers to the quantity of electric changes stored, and thus capacitance is proportional to the amount of water in the skin, a factor that commonly referred to as skin hydration. Simply put, the higher the level of skin moisture, the stronger the observed conductance signal will be (24). Baseline values were taken from ten female volunteers aged between 22 and 3 7 years, using 40-mm-diameter circular test areas on both forearms. These panelists remained at rest in a room at a temperature of 25°C, with 45-55% relative humidity, for the duration of the test. Then, each of the designated areas was treated with a 10-µl/circle of five different test formulations. Cytotoxicity assay. Human fibroblasts and keratinocytes were utilized in our assessment of the cytotoxicity of levan (5 % (w/w)). Each of the cell lines was inoculated on a 96-well plate supplemented with 100 µl of DMEM (Dulbecco's Modified Eagle Medium) con taining 10% FBS (fetal bovine serum, GIBCO BRL), with a density of 104 cells per well. These plates were then incubated for 24 hours at 3 7 ° C in an atmosphere containing 5 % CO 2 . After the addition of the levan solution, the cells were incubated for another 24 hours. The viability and proliferation of the cells were then measured via MTT assay. The MTT assay allows for convenient assays using MTT (3-(4,5-dimethylthiazol-2-yl)- 2,5-diphenyl tetrazolium bromide, yellow), which results in the generation of a water insoluble formazan dye (purple) upon bioreduction in the presence of mitochondrial dehydrogenase in viable cells (25 ). MTT solution (100 µl) was added to each well, then incubated for four hours. One hundred milliliters of 0.0lM HCI containing 10% SDS was also added. The quantity of formazan in the culture medium was determined
COSMECEUTICAL PROPERTIES OF LEVAN 399 via absorbance, measured at 570 nm with an ELISA reader. The cell viability was cal culated as Cell viability (%) = (OD 57 0(sample/OD 57 o(control)) X 100 in which the OD 57 o(sampl e ) refers to the absorbance at 5 70 nm of the cells treated with levan or SLS, and OD 57 occonrrol) is the absorbance at 5 70 nm of the negative control (non-treated cells). Cell proliferation test with 3-dimensional artificial skin (26,27). A dermal insert was con structed and cultured for three days in ascorbic acid-harboring DMEM supplemented with 10% FBS. The normal keratinocytes were seeded on this dermis at a density of 2 x 105 cells/ml and incubated for seven days, along with DMEM supplemented with ascorbic acid (50 µg/ml) as well as 10% FBS and K-SFM (1: 1). Then, the insert medium was discarded in order to induce keratinization and was cultured for a total of five days. After new medium had been added, 10 µl of SLS (sodium lauryl sulfate) was added to each of the inserts. After four hours, samples (10 µl) of each concentration were applied to the epidermis for 24 hours. One mililiter of 0.25 mg/ml MTT was applied to the insert. After an additional four hours of incubation, the MTT solution was discarded, and the MTT formazan product was extracted with 2 ml of DMSO and measured at 5 70 nm with an ELISA reader. Anti-inflammation test with 3-D artificial skin. We also carried out an interleukin-la release assay with an ELISA kit. Before MTT was added to the well plates for the cytotoxicity assay, media were collected in order to measure the release of IL-la. We used a human IL-la ELISA kit (Pierce, Rockford, IL), and E. coli-delivered recombinant human IL-la was utilized as a standard. After the human IL-la antibody was allowed to react with the sample at room temperature, the sample was treated with biotin conjugated secondary antibody. Streptavidin-HRP (horseradish peroxidase) was applied for 30 minutes, followed by treatment with trimethylbenzidine solution. The absorbance was measured at 45 0 nm using on ELISA reader. RES UL TS AND DISCUSSION CHARACTERIZATION OF LEV AN In order to determine precisely the structure of levan, we conducted a 13 C-NMR experiment using a Varian-Mercury Plus 400 spectrometer. The 13 C-NMR spectra of the levan generated by Zymomonas mobilis exhibited six primary peaks at 59.9, 63.5, 75.3, 76.3, 80.4, and 104.2 ppm. (Table I). These chemical shift values were quite similar to those of levan, much more than to those of inulin. We also observed trace peaks, but these were similar to those of inulin. Therefore, our results indicated that the structure of the polysaccharide generated by Zymomonas mobilis is primarily reminiscent of levan and is characterized by � 2➔6 linkages, with branches of� 2➔ 1 linkages (22). The measured weight-average molecular weight, as described above, was 2.25 x 106 , and the polydispersity was measured to be 2.759. PAR TI CLE SIZE MEASUREMENT Levan partially forms nanoparticles in water. The distribution of the particle size of these
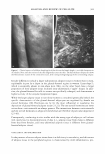
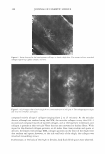
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)