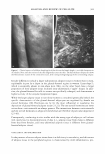
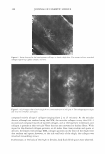
MOISTURIZER EFFICACY 431 dryness, and D.I., the effects of the test products were compared with each other and the "no product" control, using "change from baseline" data. Comparison with the "no product" control represented the effect of the product on the skin. By comparing the subsequent "no product" control readings to the baseline (Day 0), we can assess the effects of the environment on the skin. For, skin hydration, DI, and observer (visual) scoring of scaling, a two-way (time x product) repeated-measure ANOVA was run using the "change from baseline" data. When an overall significant difference (p ::::::: 0.05) was detected, the products and the untreated site were compared using the Tukey HSD test. For stained D-Squames®, statistical analyses reflected the differences between treated sites and the "no product" control site using a one-way, within-subject, ANOV A. If an overall significant difference was detected (p ::::::: 0.05), then the significance of the differences between the lotions, as well as between the untreated sites, was assessed using the Tukey HSD test. MODEL SYSTEM II. BENEFIT: PREVENTION OF DRYNESS HAND-WASH TEST Test history. Repeated exposure to surfactants and water is a major cause of dry skin and eczema. Professions such as health care, hairdressing, and food preparation have elevated rates of eczema (12). Indeed, Nilsson et al. (13) found that nurses who wash their hands frequently have double the rate of hand eczema of a balanced cohort of clerical workers. Atopy also increased the probability of the occurrence of eczema. The hand wash meth odology developed by Highley et al. (1) was selected as the model to assess the ability of moisturizers to prevent the induction of dryness. It is also possible to induce erythema and stratum cornuem damage, but this requires more handwashes than required to produce dryness (14). For this study, the design of Highley et al. was significantly updated. Instead of small study groups (n = 5), larger groups of panelists (n 2:: 25) were employed to allow for the use of better statistical methods. Furthermore, the effectiveness of multiple moisturizers was compared by trained observers, augmented with biophysical methods such as skin conductance and squamometry not available at the time of Highley' s publication. Current procedure. Thirty male and female subjects between the ages of 18 and 65 par ticipated in this study. Panelists were in good health other than having a tendency to develop dry skin on their hands. Participants were provided with Dial® liquid soap at the beginning of the study and throughout the study. Except for using Dial® liquid soap, participants were not allowed to use any other soaps, moisturizers, creams, or lotions on the backs of their hands until the study was completed. For activities in which the backs of the hands might contact another soap or detergent, panelists were to wear the rubber gloves provided. These restrictions were imposed three days before the start of the treatment phase of the study. At the beginning of the treatment phase, the baseline condition of the skin was deter mined. Panelists sat at rest in an environmentally controlled room (temperature :::::::21 °C, RH = 3 5 ± 10%) for at least 15 minutes. Once acclimated, a trained observer evaluated the skin on the back of each subject's hands using a 0-4 categorical scale for skin scaling.
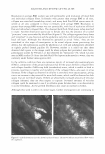
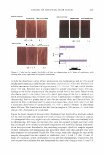
432 JOURNAL OF COSMETIC SCIENCE To qualify for the study, the backs of both hands of each participant had to have a visual scaling score of 2::1.5 and ::S2.5. On the backs of both hands of each panelist, two 2.5-cm-diameter circles were marked. The baseline condition of each site was then evaluated using a conductance meter (Skicon® 200 with an MT probe) and a trained observer. Each subject was then given Dial® liquid soap and hand-washing instructions. At the testing laboratory, subjects washed each hand with Dial® liquid soap at intervals of approximately one hour, five times each day for four days. The hand-washing instructions required subjects to wash each hand, individually, for 60 seconds, and then both hands were rinsed for 15 seconds with warm water (� 38° C). Following this, the backs of the hands were air-dried. After each of the first four ( 4) times the hands were washed and dried, a technician applied 10 µl (or approximately 1 mg/cm2) of test lotion to its assigned area. Assignment of three test products (lotions GR, W, and H) was based on a balanced complete-block design. The fourth site was left untreated ("no product" control). The fifth washing each day was used to eliminate residual test lotion that could hinder assessment. One hour after the fifth wash, during which panelists had re-acclimated in a controlled environ ment for at least 15 minutes, a clinical grader visually evaluated the hands for observable scaling, and Skicon® measurements were retaken. In doing the evaluations, the experienced evaluator terminated any panelist from the study who received a scaling score of 4 at any of the test sites. At this time, a D Squames® patch (used for the sampling of scaly skin) was collected for staining. The terminal measurements for each time were carried forward throughout the balance of the study. No additional baseline evaluations were carried out after Day 0. On Day 3, after the last hand-washing and drying procedure, D-Squames® tapes were collected for staining and color evaluation (squamometry). These D-Squames® tapes were evaluated using color imeter values (C*). Statistics. The test lotions' ability to reduce or reverse the onset of skin dryness and dehydration (evaluated by conductance) was assessed as a "change from the baseline" scores/measurements. Comparisons of this data to that of the "no product" control site represent the effects of the test lotion on the skin. The changes from baseline for the "no product" control reflect the effect of repeated hand washing on the skin. For daily skin hydration, as measured by Skicon® conductance, a two-way repeated measure ANOV A was used for analysis. The significance level was set at p ::S 0.05. Similarly, Skicon® conductance measurements of all termination scores were analyzed using a one-way, within-subject, ANOV A. The significance level was also set at p ::S 0.05. For each set of measurements, if an overall significant difference was observed (p ::S 0.05), the test products and control site were compared using the Tukey HSD test. For observer scoring of dryness, a similar statistical approach was used. The ability of the lotions to reduce the degree of damage to surface corneocytes was measured using stained D-Squames®. Day 3 results were compared with each other and with the untreated areas using a one-way, within-subject, ANOVA. The significance level was set at p ::S 0.05. When an overall significant difference was observed (p ::S 0.05 ), the Tukey HSD test was used to compare the test products and control site.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)