406 JOURNAL OF COSMETIC SCIENCE levanbiosyl transfers by levan fructotransferase of Arthrobacter ureafaciens,]. Biochern. (Tokyo), 97, 1679-1688 (1985). (9) R. Dedonder and C. Peaud-Lenoel, Studies on the levansucrase of Bacillus subtilis. I. Production of levans and levansucrase (levan-succharotransfructosidase) by cultures of Bacillus subtilis, Bull. Soc. Chim. Biol. (Paris), 39, 483--497 (1957). (10) A. Fuchs, Synthesis of levan by pseudomonads, Nature (London), l 78, 921 (1956). (11) R. Lesher, Levan production by Rothia dentocariosa and its biological activity (Ph.D. Thesis, West Virginia University, 1976). (12) J.C. C. Ribeiro, W. V. Guimaraes, A. C. Borges, D. 0. Silva, and C. D. Cruz, Levan and ethanol production by Zyrnornonas rnobilis CP4 mutants during sucrose fermentation, Rev. Microbial., 19, 196- 202 (1988). (13) H. H. Schlubach and J. Berndt, Uber Die Durch Azotobacter Chroococcurn Aus Saccharose Gebildeten Oligo- Und Polysaccharide, Liebigs Ann. Chern., 677, 172-176 (1964). (14) T. Tanaka, S. Yamamoto, S. Oi, and T. Yamamoto, Structures of heterooligosaccharides synthesized by levansucrase,J. Biochern. (Tokyo), 90, 521-526 (1981). (15) Y. Yamamoto, Y. Takahashi, M. Kawano, M. Iizuka, S. Saeki, and H. Yamaguchi, In vitro digest ibility and fermentability of levan and its hypocholesterolemic effects in rats,]. Nutr. Biochern., 10, 13-18 (1999). (16) A. Ohta, N. Osakabe, K. Yamada, Y. Saito, and H.J. Hidaka, Effect of fructooligosuccharides and other saccharides on Ca, Mg, and P absorption in rats,]. Jap. Soc. Nutr. Food Sci., 46, 123-129 (1993). (17) M. B. Roberfroid, G. R. Gibson, and N. Delzenne, The biochemistry of oligofructose, a nondigestible fiber: An approach to calculate its caloric value, Nutr. Rev., 51, 137-146 (1993). (18) G. M. T. Calazans, C. E. Lopez, R. M. 0. C. Lima, and F. P. Franca, Antitumour activities of levans produced by Zyrnornonas rnobilis strains, Biotech. Lett., 19, 19-21 (1997). (19) M. Beakers, J. Shvinka, L. Pankova, M. Laivenieks, and I. Mezhbarde, Simultaneous sucrose biocon version into ethanol and levan by Zyrnornonas mobilis, Appl. Biochem. Biotechnol., 24125, 265-274 (1990) (20) W. Hartmeier, M. Reiss, M. Heidel, and S. Marx, Biochemical and economical aspects of levan synthesis by Zymornonas rnobilis, Biocatalysis, 10, 131-136 (1994) (21) A. D. French, Chemical and physical properties of fructans,J. Plant Physiol., 134, 125-136 (1989). (22) Y. W. Han, Levan production by Bacillus polyrnyxa,]. Ind. Microbial., 4, 447-452 (1989). (23) J. Levin and H. Maibach, The correlation between transepidermal water loss and percutaneous ab sorption: An overview,]. Controlled Release, 105, 291-299 (2005). (24) G. R. Leonardi, L. R. Gaspar, and P. M. Maia Campos, Application of a non-invasive method to study the moisturizing effect of formulations containing vitamins A or E or ceramide on human skin,]. Cosmetic Sci., 53, 263-268 (2002). (25) T. Mosmann, Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays,]. Imrnunol. Methods, 65, 55-63 (1983). (26) L. P. Kamolz, M. Luegmair et al., The Viennese culture method: Cultured human epithelium obtained on a dermal matrix based on fibroblast containing fibrin glue gels, Burns, 31, 25-29 (2005). (27) M. Ponec and J. Kempenaar, Use of human skin recombinants as an in vitro model for testing the irritation potential of cutaneous irritants, Skin Pharrnacol., 8, 49-59 (1995). (28) R. A. Demel, E. Dorrepaal, M. J. M. Ebskamp, J. C. M. Smeekens, and B. D. Kruijff, Fructans interact strongly with model membranes, Biochirn. Biophys. Acta, 1375, 36--42 (1998). (29) I. J. Vereyken, V. Chupin, R. A. Demel, S. C. M. Smeekens, and B. D. Kruijff, Fructans insert between the head groups of phospholipids, Biochim. Biophys. Acta, 1510, 307-320 (2001). (30) I. K. Cohen, R. F. Diegelmann, and W. J. Lindblad, "Wound Healing: Biochemical and Clinical Aspects" (W. B. Saunders, Philadelphia, 1992). (31) P. J. Dykes, M. J. Edwards, 0. V. Merrett, H. E. Morgan, and R. Marks, In vitro reconstruction of human skin: The use of skin equivalents as potential indicators of cutaneous toxicity, Toxic. In Vitro, 5, 1-8 (1991).
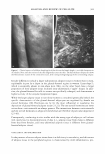
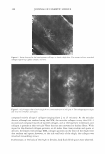
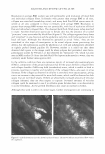
J. Cosmet. Sci., 56, 407-425 (November/December 2005) Fading of artificial hair color and its prevention by photofilters B. LOCKE and J. JACHOWICZ, International Specialty Products, Wayne, NJ 07470. Accepted for publication August 9, 2005. Synopsis Fading of artificial hair color has been investigated by simulating actual usage conditions through exposure to artificial radiation in a weatherometer, with 0.35 mW/(m2 nm) at 340 nm, for 16 to 48 hours, and by periodical washing. Hair color was produced by using commercial two-part, permanent hair dyes with light auburn, medium auburn, and dark auburn shades. Formulations based on red couplers, such as 4-amino- 2-hydroxytoluene and 1-naphtol, as well as primary intermediates, such as l-hydroxyethyl-4,5-diamino pyrazole sulfate, were employed. Results indicate that the extent of fading, as measured by the total color change parameter, dE, is greatest for colored hair subjected to both irradiation and shampooing, and significantly smaller for hair undergoing only irradiation or washing. Color loss has been also found to be dependent upon the hair type employed, with colored natural white and bleached hair undergoing much greater change than colored brown hair. It has been also shown that hair color based on pyrazole interme diates displayed the deepest fading as a result of shampooing (dE-4-6 after ten shampooings) and irradia tion/shampooing (dE-14-16 after 32 hours of light exposure and four shampooings). The contribution of UV light (UVB + UV A) to the artificial hair-color loss was found experimentally to be dependent upon the irradiation dose and varied from 63% at 16 hours of irradiation time to 27% at 48 hours of light exposure. The theoretical extent of photoprotection by a formulation was assessed by calculating the percentage of UV light it attenuates in the wavelength range from 290 nm to 400 nm. The results indicate that UVB photofilters, such as octyl methoxy cinnamate, absorb less than 25% of the total UV irradiation at concentrations as high as 30 mg/(g hair). UVA absorbers were found to be more effective, with benzo phenone-3 and benzophenone-4 absorbing about 40% of UV at the same concentration. Corresponding experimental data were in reasonable agreement with the theoretical predictions. The data are also presented for color protection with treatments containing two photo-absorbers: benzo phenone-3-ZnO benzophenone-4-ZnO octyl methoxy cinnamate-ZnO and dimethylpabaimidopropyl laurdimoni um tosy late-benzophenone-3. INTRODUCTION Hair photodamage was shown to consist of a number of concurrent processes that result in chemical and physical changes in fiber properties (Figure 1). Lipid oxidation, disulfide bond cleavage, tryptophan degradation, and cysteic acid formation lead to an increase in fiber porosity, loss of mechanical strength, and an increase in surface roughness (1-3). The most perceptible is the change in fiber coloration as a result of photoreactions of the natural pigments, either eu- or pheomelanin (4,6), as well as photodecomposition of 407
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)