J. Cosmet. Sci., 56, 449-479 (November/December 2005) Papers Presented at the Annual Scientific Seminar of the Society of Cosmetic Chemists (Friday's Program) June 2-3, 2005 Mandalay Bay Resort Las Vegas, Nevada 449
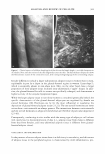
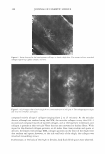
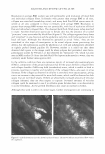
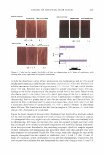
450 JOURNAL OF COSMETIC SCIENCE A MICROSCOPIC LOOK AT EMULSIONS John Carson Carson Product Development, Inc., Union City, NJ 07087 I am starting this talk at a very elementary level. Please, bear with me in this because my intention is to try to get us all "on the same page". Since we come from diverse backgrounds, this approach seemed to me to be the best way to do this. I will also proceed rapidly through the theories of dispersions and emulsions with my sincere apologies to Gibbs and Stokes and Eccleston for only cursorily referring to their important contributions to our understanding of surface activity and emulsions. Emulsions are a mixture of two insoluble liquids, where one of the liquids is dispersed in the other. If, for example, the two liquids are oil and water and the oil is dispersed within the water, then the mixture is termed an oil-in-water emulsion. Similarly, if the water is dispersed in the oil, then we have a water-in-oil emulsion. Typically, in cosmetic formulations, we deal with oil-in-water emulsions. Today we are going to look only at oil-in-water emulsions. In order to disperse one liquid within another, we must expend energy. This energy can be in the form of heat or mechanical energy or both. We are all familiar with shaking olive oil with vinegar to make a salad dressing and we have all seen that when we make a mixture this way, it is not stable and separates very quickly into two phases. If we can use a lot of energy and break up the particles very finely (such as by using a milk homogenizer), then we can make a stable dispersion that will not separate for hours or even days. Another thing that we can do to make stable emulsions is to add ingredients that reduce the amount of energy that is needed to make the dispersion. Molecules that have both water soluble portions and oil soluble sections perform this task by orienting with their oil soluble ends in the dispersed oil phase and their water soluble ends in the continuous water phase. This makes the oil more compatible with the water phase.· These materials are therefore reducing the amount of energy needed to disperse the oil in the water. In addition, these dual solubility molecules (called surfactants) define the size of the dispersed oil droplets. Smaller droplets make more stable dispersions (Stokes equation) Where: V(sedimentation) = 9 (�p) d 2 18 (�) g = gravitation constant �p = density difference d = droplet diameter � = viscosity But, not all surfactants are created equal. They have different oil soluble ends (and therefore affinities for the oil phase) and they have different water soluble ends (again with differing affinities for the water phase) that greatly affect the oil droplet's compatibility with the water phase, the oil droplet's size and the formation of "surfactant hydrate structures" in the water phase. These structures are important in helping to stabilize emulsions because they help to prevent the close approach and coalescence of the oil droplets. A common way to form these surfactant hydrate structures is to combine a water soluble surfactant (such as a soap) with a low water solubility surfactant (such as a fatty alcohol or a fatty monoglyceride).
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)